Abstract
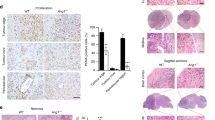
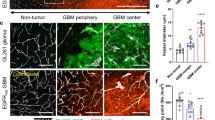
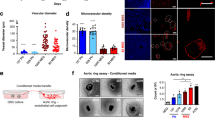
Individuals bearing germ line mutations in the Von Hippel–Lindau (VHL) tumor suppressor gene are predisposed to the development of highly angiogenic tumors. This is correlated with an increased expression of the angiogenic factor vascular endothelial growth factor (VEGF) in these tumors, which is in part caused by elevated expression of the HIF-1 hypoxia inducible transcription factors. We created malignant astrocytes with genetic deletions of the VHL gene and implanted them in subcutaneous and intracranial sites; these sites are respectively vessel poor and vessel-rich tissues. When grown in a vessel poor site, VEGF expression in VHL null cells was important for both vascularization and tumor growth. However, when the same cells are grown in the vessel-rich intracranial environment, loss of VEGF expression reduces vascularization, but does not affect tumor growth. This indicates that antiangiogenic therapies for tumors that express high levels of angiogenic factors such as VEGF may vary in their efficacy, with potentially lowered effectiveness in sites, such as the brain, that are inherently vessel rich.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Benjamin LE, Keshet E . (1997). Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci USA 94: 8761–8766.
Bergers G, Benjamin LE . (2003). Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3: 401–410.
Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP et al. (2003). The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4: 133–146.
Carroll VA, Ashcroft M . (2006). Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel–Lindau function: implications for targeting the HIF pathway. Cancer Res 66: 6264–6270.
Flamme I, Krieg M, Plate KH . (1998). Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2alpha. Am J Pathol 153: 25–29.
Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L et al. (1999). VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159.
Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS . (1999). Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res 59: 1592–1598.
Haase VH, Glickman JN, Socolovsky M, Jaenisch R . (2001). Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc Natl Acad Sci USA 98: 1583–1588.
Hansel DE . (2006). Genetic alterations and histopathologic findings in familial renal cell carcinoma. Histol Histopathol 21: 437–444.
Harris AL . (2000). von Hippel–Lindau syndrome: target for anti-vascular endothelial growth factor (VEGF) receptor therapy. Oncologist 5 (Suppl 1): 32–36.
Hasselblatt M, Jeibmann A, Gerss J, Behrens C, Rama B, Wassmann H et al. (2005). Cellular and reticular variants of haemangioblastoma revisited: a clinicopathologic study of 88 cases. Neuropathol Appl Neurobiol 31: 618–622.
Kaelin Jr WG . (2002). Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2: 673–682.
Koochekpour S, Jeffers M, Wang PH, Gong C, Taylor GA, Roessler LM et al. (1999). The von Hippel–Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor-induced invasion and branching morphogenesis in renal carcinoma cells. Mol Cell Biol 19: 5902–5912.
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM et al. (2003). von Hippel–Lindau disease. Lancet 361: 2059–2067.
Los M, Zeamari S, Foekens JA, Gebbink MF, Voest EE . (1999). Regulation of the urokinase-type plasminogen activator system by the von Hippel–Lindau tumor suppressor gene. Cancer Res 59: 4440–4445.
Mack FA, Patel JH, Biju MP, Haase VH, Simon MC . (2005). Decreased growth of Vhl−/− fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol Cell Biol 25: 4565–4578.
Mack FA, Rathmell WK, Arsham AM, Gnarra J, Keith B, Simon MC . (2003). Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell 3: 75–88.
Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM et al. (2002). HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1: 459–468.
Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH . (2005). Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel–Lindau disease-associated vascular tumors in mice. Mol Cell Biol 25: 3163–3172.
Rathmell WK, Acs G, Simon MC, Vaughn DJ . (2004). HIF transcription factor expression and induction of hypoxic response genes in a retroperitoneal angiosarcoma. Anticancer Res 24: 167–169.
Ryan HE, Lo J, Johnson RS . (1998). HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015.
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM et al. (2000). Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 60: 4010–4015.
Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W et al. (2003). HIF 1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development 130: 1713–1724.
Staehler M, Rohrmann K, Haseke N, Stief CG, Siebels M . (2005). Targeted agents for the treatment of advanced renal cell carcinoma. Curr Drug Targets 6: 835–846.
Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W . (2003). Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature 425: 307–311.
Tang N, Mack F, Haase VH, Simon MC, Johnson RS . (2006). pVHL function is essential for endothelial extracellular matrix deposition. Mol Cell Biol 26: 2519–2530.
Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP et al. (2004). Loss of HIF-alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6: 485–495.
Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E et al. (2003). The hypoxia-inducible factor-1 alpha is a negative factor for tumour therapy. Oncogene 22: 3213–3220.
Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA et al. (2005). Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel–Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res 65: 6178–6188.
Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL . (2000). Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 88: 2606–2618.
Acknowledgements
BB wishes to thank Dr Veronica Sanchez for helpful comments on the paper. GB acknowledges support by the Goldhirsh Foundation, VHH by NIH CA 100787 and RSJ by NIH CA082515.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Blouw, B., Haase, V., Song, H. et al. Loss of vascular endothelial growth factor expression reduces vascularization, but not growth, of tumors lacking the Von Hippel–Lindau tumor suppressor gene. Oncogene 26, 4531–4540 (2007). https://doi.org/10.1038/sj.onc.1210249
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210249