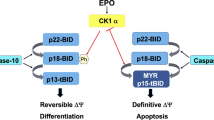
Abstract
Imatinib targets the Bcr-Abl oncogene that causes chronic myelogenous leukemia (CML) in humans. Recently, we demonstrated that besides triggering apoptosis in K562 cells, imatinib also mediated their erythroid differentiation. Although both events appear to proceed concomitantly, it is not known at present whether or not imatinib-induced apoptosis and differentiation are interdependent processes. Hence, we investigated the requirements for Bcr-Abl inhibitor-mediated apoptosis and erythroid differentiation in several established and engineered CML cell lines. Imatinib triggered apoptosis and erythroid differentiation of different CML cell lines, but only apoptosis exhibited sensitivity to ZVAD-fmk inhibition. Conversely, the p38 mitogen-activated protein (MAP) kinase inhibitor, SB202190, significantly slowed down erythroid differentiation without affecting caspase activation. Furthermore, imatinib and PD166326, another Bcr-Abl inhibitory molecule, triggered erythroid differentiation of K562 cell clones, nevertheless resistant to Bcr-Abl inhibitor-induced apoptosis. Finally, short hairpin RNA inhibitor (shRNAi) silencing of caspase 3 efficiently inhibited caspase activity but had no effect on erythroid differentiation, whereas silencing of Bcr-Abl mimicked imatinib or PD166326 treatment, leading to increased apoptosis and erythroid differentiation of K562 cells. Taken together, our findings not only demonstrate that Bcr-Abl inhibitor-mediated apoptosis and differentiation are fully distinguishable events, but also that caspases are dispensable for erythroid differentiation of established CML cell lines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Belhacene N, Maulon L, Guerin S, Ricci JE, Mari B, Colin Y et al. (1998). Differential expression of the Kell blood group and CD10 antigens: two related membrane metallopeptidases during differentiation of K562 cells by phorbol ester and hemin. FASEB J 12: 531–539.
Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ et al. (2000). Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295: 139–145.
Carlile GW, Smith DH, Wiedmann M . (2004). Caspase-3 has a nonapoptotic function in erythroid maturation. Blood 103: 4310–4316.
Deininger MW, Goldman JM, Melo JV . (2000). The molecular biology of chronic myeloid leukemia. Blood 96: 3343–3356.
Dorsey JF, Cunnick JM, Mane SM, Wu J . (2002). Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl(+) K562 leukemic cells by Gab2. Blood 99: 1388–1397.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ . (2000). Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 96: 925–932.
Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, Luciano F et al. (2004). Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene 23: 7863–7873.
Huron DR, Gorre ME, Kraker AJ, Sawyers CL, Rosen N, Moasser MM . (2003). A novel pyridopyrimidine inhibitor of abl kinase is a picomolar inhibitor of Bcr-abl-driven K562 cells and is effective against STI571-resistant Bcr-abl mutants. Clin Cancer Res 9: 1267–1273.
Jacquel A, Herrant M, Defamie V, Belhacene N, Colosetti P, Marchetti S et al. (2006). A survey of the signaling pathways involved in megakaryocytic differentiation of the human K562 leukemia cell line by molecular and c-DNA array analysis. Oncogene 25: 781–794.
Jacquel A, Herrant M, Legros L, Belhacene N, Luciano F, Pages G et al. (2003). Imatinib induces mitochondria-dependent apoptosis of the Bcr-Abl-positive K562 cell line and its differentiation toward the erythroid lineage. FASEB J 17: 2160–2162.
Kawano T, Horiguchi-Yamada J, Iwase S, Furukawa Y, Kano Y, Yamada H . (2004). Inactivation of ERK accelerates erythroid differentiation of K562 cells induced by herbimycin A and STI571 while activation of MEK1 interferes with it. Mol Cell Biochem 258: 25–33.
Kohmura K, Miyakawa Y, Kawai Y, Ikeda Y, Kizaki M . (2004). Different roles of p38 MAPK and ERK in STI571-induced multi-lineage differentiation of K562 cells. J Cell Physiol 198: 370–376.
Kolbus A, Pilat S, Husak Z, Deiner EM, Stengl G, Beug H et al. (2002). Raf-1 antagonizes erythroid differentiation by restraining caspase activation. J Exp Med 196: 1347–1353.
Krauss SW, Lo AJ, Short SA, Koury MJ, Mohandas N, Chasis JA . (2005). Nuclear substructure reorganization during late-stage erythropoiesis is selective and does not involve caspase cleavage of major nuclear substructural proteins. Blood 106: 2200–2205.
Kuzelova K, Grebenova D, Marinov I, Hrkal Z . (2005). Fast apoptosis and erythroid differentiation induced by imatinib mesylate in JURL-MK1 cells. J Cell Biochem 95: 268–280.
Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C . (2005). Vital functions for lethal caspases. Oncogene 24: 5137–5148.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al. (2003). Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348: 994–1004.
Okada M, Adachi S, Imai T, Watanabe K, Toyokuni SY, Ueno M et al. (2004). A novel mechanism for imatinib mesylate-induced cell death of BCR-ABL-positive human leukemic cells: caspase-independent, necrosis-like programmed cell death mediated by serine protease activity. Blood 103: 2299–2307.
Parmar S, Katsoulidis E, Verma A, Li Y, Sassano A, Lal L et al. (2004). Role of the p38 mitogen-activated protein kinase pathway in the generation of the effects of imatinib mesylate (STI571) in BCR-ABL-expressing cells. J Biol Chem 279: 25345–25352.
Perfettini JL, Kroemer G . (2003). Caspase activation is not death. Nat Immunol 4: 308–310.
Pettiford SM, Herbst R . (2003). The protein tyrosine phosphatase HePTP regulates nuclear translocation of ERK2 and can modulate megakaryocytic differentiation of K562 cells. Leukemia 17: 366–378.
Racke FK, Wang D, Zaidi Z, Kelley J, Visvader J, Soh JW et al. (2001). A potential role for protein kinase C-epsilon in regulating megakaryocytic lineage commitment. J Biol Chem 276: 522–528.
Rangatia J, Bonnet D . (2005). Transient or long-term silencing of BCR-ABL alone induces cell cycle and proliferation arrest, apoptosis and differentiation. Leukemia 20: 68–76.
Roskoski Jr R . (2003). STI-571: an anticancer protein-tyrosine kinase inhibitor. Biochem Biophys Res Commun 309: 709–717.
Scherr M, Battmer K, Winkler T, Heidenreich O, Ganser A, Eder M . (2003). Specific inhibition of bcr-abl gene expression by small interfering RNA. Blood 101: 1566–1569.
Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA . (2004). JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 18: 189–218.
van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D et al. (2003). Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4: 609–615.
Withey JM, Harvey AJ, Crompton MR . (2005). RNA interference targeting of Bcr-Abl increases chronic myeloid leukemia cell killing by 17-allylamino-17-demethoxygeldanamycin. Leuk Res 30: 553–560.
Wohlbold L, Van der Kuip H, Miething C, Vornlocher HP, Knabbe C, Duyster J et al. (2003). Inhibition of bcr-abl gene expression by small interfering RNA sensitizes for imatinib mesylate (STI571). Blood 102: 2236–2239.
Wolff NC, Veach DR, Tong WP, Bornmann WG, Clarkson B, Ilaria Jr RL . (2005). PD166326, a novel tyrosine kinase inhibitor, has greater antileukemic activity than imatinib mesylate in a murine model of chronic myeloid leukemia. Blood 105: 3995–4003.
Wong S, Witte ON . (2004). The BCR-ABL story: bench to bedside and back. Annu Rev Immunol 22: 247–306.
Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F et al. (2001). Caspase activation is required for terminal erythroid differentiation. J Exp Med 193: 247–254.
Acknowledgements
We are indebted to Dr Hans Clever for the kind gift of the pTER vector for inducible expression of shRNAi. We thank Novartis for the kind gift of imatinib mesylate and Pfizer for that of PD166326. SM and AJ are recipients of fellowships from the Fondation contre la Leucémie and the Ligue Nationale contre le Cancer, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacquel, A., Colosetti, P., Grosso, S. et al. Apoptosis and erythroid differentiation triggered by Bcr-Abl inhibitors in CML cell lines are fully distinguishable processes that exhibit different sensitivity to caspase inhibition. Oncogene 26, 2445–2458 (2007). https://doi.org/10.1038/sj.onc.1210034
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210034
Keywords
This article is cited by
-
Cabozantinib promotes erythroid differentiation in K562 erythroleukemia cells through global changes in gene expression and JNK activation
Cancer Gene Therapy (2022)
-
Pharmacologic targeting of the P-TEFb complex as a therapeutic strategy for chronic myeloid leukemia
Cell Communication and Signaling (2021)
-
A systems biology pipeline identifies regulatory networks for stem cell engineering
Nature Biotechnology (2019)
-
Suppression of c-Myc induces apoptosis via an AMPK/mTOR-dependent pathway by 4-O-methyl-ascochlorin in leukemia cells
Apoptosis (2016)
-
The caspase 6 derived N-terminal fragment of DJ-1 promotes apoptosis via increased ROS production
Cell Death & Differentiation (2012)