Abstract
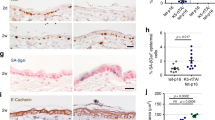
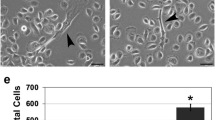
Epidermal growth factor receptor (EGFR) overexpression and activation is critical in the initiation and progression of cancers, especially those of epithelial origin. EGFR activation is associated with the induction of divergent signal transduction pathways and a gamut of cellular processes; however, the cell-type and tissue-type specificity conferred by certain pathways remains to be elucidated. In the context of the esophageal epithelium, a prototype stratified squamous epithelium, EGFR overexpression is relevant in the earliest events of carcinogenesis as modeled in a three-dimensional organotypic culture system. We demonstrate that the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, and not the MEK/MAPK (mitogen-activated protein kinase) pathway, is preferentially activated in EGFR-mediated esophageal epithelial hyperplasia, a premalignant lesion. The hyperplasia was abolished with direct inhibition of PI3K and of AKT but not with inhibition of the MAPK pathway. With the introduction of an inducible AKT vector in both primary and immortalized esophageal epithelial cells, we find that AKT overexpression and activation is permissive for complete epithelial formation in organotypic culture, but imposes a growth constraint in cells grown in monolayer. In organotypic culture, AKT mediates changes related to cell shape and size with an expansion of the differentiated compartment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K et al. (2003). Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem 278: 1824–1830.
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA . (2004). The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene 23: 8571–8580.
Chan KS, Carbajal S, Kiguchi K, Clifford J, Sano S, DiGiovanni J . (2004). Epidermal growth factor receptor-mediated activation of Stat3 during multistage skin carcinogenesis. Cancer Res 64: 2382–2389.
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y et al. (1997). Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241.
Debnath J, Brugge JS . (2005). Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 5: 675–688.
Debnath J, Walker SJ, Brugge JS . (2003). Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol 163: 315–326.
Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B et al. (2003). Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res 1: 729–738.
Hunter T . (2000). Signaling – 2000 and beyond. Cell 100: 113–127.
Itoiz ME, Conti CJ, Lanfranchi HE, Mamrack M, Klein-Szanto AJ . (1985). Immunohistochemical detection of fillaggrin in preneoplastic and neoplastic lesions of the human oral mucosa. Am J Pathol 119: 456–461.
Janda E, Litos G, Grunert S, Downward J, Beug H . (2002). Oncogenic Ras/Her-2 mediate hyperproliferation of polarized epithelial cells in 3D cultures and rapid tumor growth via the PI3K pathway. Oncogene 21: 5148–5159.
Janes SM, Ofstad TA, Campbell DH, Watt FM, Prowse DM . (2004). Transient activation of FOXN1 in keratinocytes induces a transcriptional programme that promotes terminal differentiation: contrasting roles of FOXN1 and Akt. J Cell Sci 117: 4157–4168.
Kamei T, Jones SR, Chapman BM, KL MC, Dai G, Soares MJ . (2002). The phosphatidylinositol 3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol Endocrinol 16: 1469–1481.
Kandel ES, Hay N . (1999). The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res 253: 210–229.
Kim YT, Park SW, Kim JW . (2002). Correlation between expression of EGFR and the prognosis of patients with cervical carcinoma. Gynecol Oncol 87: 84–89.
Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A et al. (2005). AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 207: 139–146.
Kohn AD, Barthel A, Kovacina KS, Boge A, Wallach B, Summers SA et al. (1998). Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem 273: 11937–11943.
Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM . (1999). Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398: 630–634.
Li W, Marshall C, Mei L, Dzubow L, Schmults C, Dans M et al. (2005). Srcasm modulates EGF and Src-kinase signaling in keratinocytes. J Biol Chem 280: 6036–6046.
Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW . (1998). Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 12: 3008–3019.
Lo HW, Hsu SC, Hung MC . (2006). EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat 95: 211–218.
Mandard AM, Hainaut P, Hollstein M . (2000). Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res 462: 335–342.
Mayo LD, Donner DB . (2001). A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 98: 11598–11603.
Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I . (2004). Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBOJ 23: 212–220.
Murphy GF . (2005). Histology of the skin. In: Elder DE, Elenitsas R, Johnson B and Murphy GF (eds). Lever's Histopathology of the Skin, 4th edn. Lippincott: Philadelphia, p 13.
Nelson CM, Bissell MJ . (2005). Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol 15: 342–352.
Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H . (2000). Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem 275: 30934–30942.
Okano J, Snyder L, Rustgi AK . (2003). Genetic alterations in esophageal cancer. Methods Mol Biol 222: 131–145.
Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A . (2001). The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 8: 11–31.
Scholle F, Bendt KM, Raab-Traub N . (2000). Epstein–Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol 74: 10681–10689.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW . (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602.
Sugatani T, Hruska KA . (2005). Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem 280: 3583–3589.
Xing D, Orsulic S . (2005). A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc Natl Acad Sci USA 102: 6936–6941.
Zahir N, Weaver VM . (2004). Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev 14: 71–80.
Acknowledgements
This work was supported by the NIH/NCI Grant P01 DE12467 (AKR, KO, TO, HN, CA, AK, AKS, MH, WED), NIH/NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30 DK50306), and the Morphology, Molecular Biology, Mouse and Cell Culture Core Facilities, NIH/NCI Grants CA 80999 and CA 25874 (both to MH), K01-DK066205 9 (HN), and F32-CA108657 and the AGA/FDHN Research Scholar Award (both to CA). We thank members of the Rustgi lab (Therese Deramaudt, Cameron Johnstone, Carmen Michaylira, Doug Stairs and Ben Rhoades) for discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Oyama, K., Okawa, T., Nakagawa, H. et al. AKT induces senescence in primary esophageal epithelial cells but is permissive for differentiation as revealed in organotypic culture. Oncogene 26, 2353–2364 (2007). https://doi.org/10.1038/sj.onc.1210025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210025
Keywords
This article is cited by
-
Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses?
Cellular and Molecular Life Sciences (2017)
-
LRRC31 is induced by IL-13 and regulates kallikrein expression and barrier function in the esophageal epithelium
Mucosal Immunology (2016)
-
Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities
Oncogene (2015)
-
Inhibition of heat shock protein 90 suppresses squamous carcinogenic progression in a mouse model of esophageal cancer
Journal of Cancer Research and Clinical Oncology (2015)
-
Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis
Mucosal Immunology (2014)