Abstract
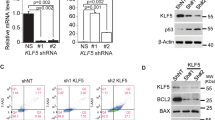
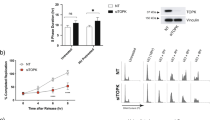
In response to γ-radiation-induced DNA damage, organisms either activate cell cycle checkpoint and repair machinery or undergo apoptosis to eliminate damaged cells. Although previous studies indicated that the tumor suppressor p53 is critically involved in mediating both responses, how a cell decides which pathway to take is not well established. The zinc-finger-containing transcription factor, Krüppel-like factor 4 (KLF4), is a crucial mediator for the checkpoint functions of p53 after γ-irradiation and does so by inhibiting the transition from the G1 to S and G2 to M phases of the cell cycle. Here, we determined the role of KLF4 in modulating the apoptotic response following γ-irradiation. In three independent cell systems including colorectal cancer cells and mouse embryo fibroblasts in which expression of KLF4 could be manipulated, we observed that γ-irradiated cells underwent apoptosis if KLF4 was absent. In the presence of KLF4, the degree of apoptosis was significantly reduced and cells resorted to checkpoint arrest. The mechanism by which KLF4 accomplished this antiapoptotic effect is by activating expression of the cell cycle arrest gene, p21WAF1/CIP1, and by inhibiting the ability of p53 to transactivate expression of the proapoptotic gene, BAX. Results of our study illustrate an unexpected antiapoptotic function of KLF4, heretofore considered a tumor suppressor in colorectal cancer, and suggest that KLF4 may be an important determinant of cell fate following γ-radiation-induced DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- bp:
-
base pair
- ChIP:
-
chromatin immunoprecipitation
- DMEM:
-
Dulbecco's modified Eagle's medium
- FACS:
-
fluorescence-activated cell sorting
- FBS:
-
fetal bovine serum
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- KLF4:
-
Krüppel-like factor 4
- MEFs:
-
mouse embryo fibroblasts
- nt:
-
nucleotide
- PBS:
-
phosphate-buffered saline
- shRNA:
-
small hairpin RNA
References
Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG et al. (2004). Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol 24: 1219–1231.
Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L et al. (1999). Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest 104: 263–269.
Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B . (2000). Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev 14: 1584–1588.
Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G et al. (2001). Kruppel-like factor 4 (gut-enriched Kruppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem 276: 30423–30428.
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML et al. (1993). Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362: 849–852.
Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW . (2001). Expression of the gut-enriched Kruppel-like factor (Kruppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene 20: 4884–4890.
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825.
Erster S, Mihara M, Kim RH, Petrenko O, Moll UM . (2004). In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol 24: 6728–6741.
Fei P, El-Deiry WS . (2003). P53 and radiation responses. Oncogene 22: 5774–5783.
Foster KW, Liu Z, Nail CD, Li X, Fitzgerald TJ, Bailey SK et al. (2005). Induction of KLF4 in basal keratinocytes blocks the proliferation–differentiation switch and initiates squamous epithelial dysplasia. Oncogene 24: 1491–1500.
Foster KW, Ren S, Louro ID, Lobo-Ruppert SM, McKie-Bell P, Grizzle W et al. (1999). Oncogene expression cloning by retroviral transduction of adenovirus E1A-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zinc finger protein GKLF. Cell Growth Differ 10: 423–434.
Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B . (1996). A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem 271: 31384–31390.
Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S et al. (1997). 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell 1: 3–11.
Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A et al. (2000). Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc Natl Acad Sci USA 97: 7301–7306.
Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG et al. (2005). Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology 128: 935–945.
Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW et al. (2002). The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129: 2619–2628.
Lewanski CR, Gullick WJ . (2001). Radiotherapy and cellular signalling. Lancet Oncol 2: 366–370.
Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK et al. (2004). Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet 36: 63–68.
Lowe SW, Ruley HE, Jacks T, Housman DE . (1993a). p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T . (1993b). p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849.
Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P et al. (2003). p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11: 577–590.
Miyashita T, Reed JC . (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299.
Rowland BD, Bernards R, Peeper DS . (2005). The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol 7: 1074–1082.
Rowland BD, Peeper DS . (2006). KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer 6: 11–23.
Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW . (2002). Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1: 289–298.
Schumacher B, Hofmann K, Boulton S, Gartner A . (2001). The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr Biol 11: 1722–1727.
Shields JM, Christy RJ, Yang VW . (1996). Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem 271: 20009–20017.
Shields JM, Yang VW . (1997). Two potent nuclear localization signals in the gut-enriched Kruppel-like factor define a subfamily of closely related Kruppel proteins. J Biol Chem 272: 18504–18507.
Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T et al. (1994). p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78: 703–711.
Vogelstein B, Lane D, Levine AJ . (2000). Surfing the p53 network. Nature 408: 307–310.
Waldman T, Kinzler KW, Vogelstein B . (1995). p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res 55: 5187–5190.
Waldman T, Lengauer C, Kinzler KW, Vogelstein B . (1996). Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381: 713–716.
Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B et al. (1997). Cell-cycle arrest versus cell death in cancer therapy. Nat Med 3: 1034–1036.
Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC et al. (2005). Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res 65: 2746–2754.
Yoon HS, Chen X, Yang VW . (2003). Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem 278: 2101–2105.
Yoon HS, Ghaleb AM, Nandan MO, Hisamuddin IM, Dalton WB, Yang VW . (2005). Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene 24: 4017–4025.
Yoon HS, Yang VW . (2004). Requirement of Kruppel-like factor 4 in preventing entry into mitosis following DNA damage. J Biol Chem 279: 5035–5041.
Yu J, Tiwari S, Steiner P, Zhang L . (2003). Differential apoptotic response to the proteasome inhibitor Bortezomib [VELCADE, PS-341] in Bax-deficient and p21-deficient colon cancer cells. Cancer Biol Ther 2: 694–699.
Yu J, Zhang L . (2005). The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331: 851–858.
Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH et al. (2000). The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem 275: 18391–18398.
Zhang W, Shields JM, Sogawa K, Fujii-Kuriyama Y, Yang VW . (1998). The gut-enriched Kruppel-like factor suppresses the activity of the CYP1A1 promoter in an Sp1-dependent fashion. J Biol Chem 273: 17917–17925.
Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, Yang VW . (2004). Identification of Kruppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene 23: 395–402.
Acknowledgements
We thank B Vogelstein for providing the pC53-SN3 expression construct and the HCT116BAX−/− and p21WAF1/CIP1−/− cell lines. This work was in part supported by grants from the National Institutes of Health (DK52230, DK64399 and CA84197). VWY is the recipient of a Georgia Cancer Coalition Distinguished Cancer Clinician Scientist Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Ghaleb, A., Katz, J., Kaestner, K. et al. Krüppel-like factor 4 exhibits antiapoptotic activity following γ-radiation-induced DNA damage. Oncogene 26, 2365–2373 (2007). https://doi.org/10.1038/sj.onc.1210022
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210022
Keywords
This article is cited by
-
KLF4-mediated upregulation of the NKG2D ligand MICA in acute myeloid leukemia: a novel therapeutic target identified by enChIP
Cell Communication and Signaling (2023)
-
Identification of genetic associations and functional SNPs of bovine KLF6 gene on milk production traits in Chinese holstein
BMC Genomic Data (2023)
-
MiR-7-5p/KLF4 signaling inhibits stemness and radioresistance in colorectal cancer
Cell Death Discovery (2023)
-
Therapeutic Targeting of Krüppel-Like Factor 4 and Its Pharmacological Potential in Parkinson’s Disease: a Comprehensive Review
Molecular Neurobiology (2023)
-
Genome-wide association study identifies genetic susceptibility loci and pathways of radiation-induced acute oral mucositis
Journal of Translational Medicine (2020)