Abstract
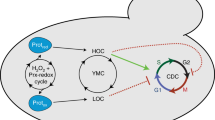
In recent years, the intracellular oxidation–reduction (redox) state has gained increasing attention as a critical mediator of cell signaling, gene expression changes and proliferation. This review discusses the evidence for a redox cycle (i.e., fluctuation in the cellular redox state) regulating the cell cycle. The presence of redox-sensitive motifs (cysteine residues, metal co-factors in kinases and phosphatases) in several cell cycle regulatory proteins indicate periodic oscillations in intracellular redox state could play a central role in regulating progression from G0/G1 to S to G2 and M cell cycle phases. Fluctuations in the intracellular redox state during cell cycle progression could represent a fundamental mechanism linking oxidative metabolic processes to cell cycle regulatory processes. Proliferative disorders are central to a variety of human pathophysiological conditions thought to involve oxidative stress. Therefore, a more complete understanding of redox control of the cell cycle could provide a biochemical rationale for manipulating aberrant cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Abate C, Luk D, Gagne E, Roeder RG, Curran T . (1990). Fos and jun cooperate in transcriptional regulation via heterologous activation domains. Mol Cell Biol 10: 5532–5535.
Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB et al. (1997). Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272: 217–221.
Bloomfield G, Pears C . (2003). Superoxide signalling required for multicellular development of Dictyostelium. J Cell Sci 116 (Part 16): 3387–3397.
Bokoch GM, Knaus UG . (2003). NADPH oxidases: not just for leukocytes anymore!. Trends Biochem Sci 28: 502–508.
Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR . (1999). Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem 274: 20017–20026.
Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH . (1991). Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351: 714–718.
Brown MR, Miller Jr FJ, Li WG, Ellingson AN, Mozena JD, Chatterjee P et al. (1999). Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 85: 524–533.
Burch PM, Heintz NH . (2005). Redox regulation of cell-cycle re-entry: cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid Redox Signal 7: 741–751.
Burdon RH, Gill V . (1993). Cellularly generated active oxygen species and HeLa cell proliferation. Free Radical Res Commun 19: 203–213.
Burdon RH, Gill V, Rice-Evans C . (1989). Cell proliferation and oxidative stress. Free Radical Res Commun 7: 149–159.
Burdon RH, Gill V, Rice-Evans C . (1990). Oxidative stress and tumour cell proliferation. Free Radical Res Commun 11: 65–76.
Burdon RH, Rice-Evans C . (1989). Free radicals and the regulation of mammalian cell proliferation. Free Radical Res Commun 6: 345–358.
Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG . (2002). Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem 277: 25370–25376.
Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G et al. (2003). Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol 161: 933–944.
Conour JE, Graham WV, Gaskins HR . (2004). A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol Genom 18: 196–205.
Demasi M, Silva GM, Netto LE . (2003). 20S proteasome from Saccharomyces cerevisiae is responsive to redox modifications and is S-glutathionylated. J Biol Chem 278: 679–685.
DeYulia Jr. GJ, Carcamo JM, Borquez-Ojeda O, Shelton CC, Golde DW . (2005). Hydrogen peroxide generated extracellularly by receptor–ligand interaction facilitates cell signaling. Proc Natl Acad Sci USA 102: 5044–5049.
Di Leonardo A, Linke SP, Clarkin K, Wahl GM . (1994). DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 8: 2540–2551.
Di Mascio P, Murphy ME, Sies H . (1991). Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr 53 (Suppl 1): 194S–200S.
Diehl JA, Cheng M, Roussel MF, Sherr CJ . (1998). Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511.
Dunphy WG, Kumagai A . (1991). The cdc25 protein contains an intrinsic phosphatase activity. Cell 67: 189–196.
Esposito F, Cuccovillo F, Vanoni M, Cimino F, Anderson CW, Appella E et al. (1997). Redox-mediated regulation of p21(waf1/cip1) expression involves a post-transcriptional mechanism and activation of the mitogen-activated protein kinase pathway. Eur J Biochem 245: 730–737.
Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T . (1983). Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33: 389–396.
Fernandes AP, Holmgren A . (2004). Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6: 63–74.
Fischle W, Wang Y, Allis CD . (2003). Binary switches and modification cassettes in histone biology and beyond. Nature 425: 475–479.
Folz RJ, Crapo JD . (1994). Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics 22: 162–171.
Fridovich I . (1989). Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 264: 7761–7764.
Gamou S, Shimizu N . (1995). Hydrogen peroxide preferentially enhances the tyrosine phosphorylation of epidermal growth factor receptor. FEBS Lett 357: 161–164.
Go YM, Gipp JJ, Mulcahy RT, Jones DP . (2004). H2O2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem 279: 5837–5845.
Goswami PC, Roti Roti JL, Hunt CR . (1996). The cell cycle-coupled expression of topoisomerase IIalpha during S phase is regulated by mRNA stability and is disrupted by heat shock or ionizing radiation. Mol Cell Biol 16: 1500–1508.
Goswami PC, Sheren J, Albee LD, Parsian A, Sim JE, Ridnour LA et al. (2000). Cell cycle-coupled variation in topoisomerase IIalpha mRNA is regulated by the 3′-untranslated region. Possible role of redox-sensitive protein binding in mRNA accumulation. J Biol Chem 275: 38384–38392.
Grana X, Reddy EP . (1995). Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 11: 211–219.
Halliwell B, Gutteridge JM . (1992). Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett 307: 108–112.
Halliwell B, Gutteridge JMC . (1999). Free Radicals in Biology and Medicine 3rd edn. Oxford University Press: New York.
Hartwell LH, Culotti J, Reid B . (1970). Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci USA 66: 352–359.
Havre PA, O’Reilly S, McCormick JJ, Brash DE . (2002). Transformed and tumor-derived human cells exhibit preferential sensitivity to the thiol antioxidants, N-acetyl cysteine and penicillamine. Cancer Res 62: 1443–1449.
Humphries KM, Deal MS, Taylor SS . (2005). Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J Biol Chem 280: 2750–2758.
Hutter DE, Till BG, Greene JJ . (1997). Redox state changes in density-dependent regulation of proliferation. Exp Cell Res 232: 435–438.
Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell Jr TR, Gong J et al. (1997). Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem 272: 28218–28226.
Janssen YM, Heintz NH, Mossman BT . (1995). Induction of c-fos and c-jun proto-oncogene expression by asbestos is ameliorated by N-acetyl-L-cysteine in mesothelial cells. Cancer Res 55: 2085–2089.
Jonas CR, Ziegler TR, Gu LH, Jones DP . (2002). Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radical Biol Med 33: 1499–1506.
Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade Jr JM, Kirlin WG . (2004). Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 18: 1246–1248.
Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS et al. (2000). Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol 165: 2190–2197.
Kawamura N . (1960). Cytochemical and quantitative study of protein-bound sulfhydryl and disulfide groups in eggs of Arbacia during the first cleavage. Exp Cell Res 20: 127–138.
Kim KY, Rhim T, Choi I, Kim SS . (2001). N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem 276: 40591–40598.
Klebanoff SJ . (2005). Myeloperoxidase: friend and foe. J Leukocyte Biol 77: 598–625.
Knapp LT, Klann E . (2000). Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem 275: 24136–24145.
Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Fujita Y et al. (2004). Antioxidant effects of stereoisomers of N-acetylcysteine (NAC), L-NAC and D-NAC, on angiotensin II-stimulated MAP kinase activation and vascular smooth muscle cell proliferation. J Pharmacol Sci 95: 483–486.
Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A et al. (2005). Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 65: 948–956.
Li N, Oberley TD . (1998). Modulation of antioxidant enzymes, reactive oxygen species, and glutathione levels in manganese superoxide dismutase-overexpressing NIH/3T3 fibroblasts during the cell cycle. J Cell Physiol 177: 148–160.
Liberto M, Cobrinik D . (2000). Growth factor-dependent induction of p21(CIP1) by the green tea polyphenol, epigallocatechin gallate. Cancer Lett 154: 151–161.
Limoli CL, Rola R, Giedzinski E, Mantha S, Huang TT, Fike JR . (2004). Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci USA 101: 16052–16057.
Liu M, Wikonkal NM, Brash DE . (1999). Induction of cyclin-dependent kinase inhibitors and G(1) prolongation by the chemopreventive agent N-acetylcysteine. Carcinogenesis 20: 1869–1872.
Lo YY, Cruz TF . (1995). Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem 270: 11727–11730.
Manome Y, Datta R, Taneja N, Shafman T, Bump E, Hass R et al. (1993). Coinduction of c-jun gene expression and internucleosomal DNA fragmentation by ionizing radiation. Biochemistry 32: 10607–10613.
Martinez Munoz C, Post JA, Verkleij AJ, Verrips CT, Boonstra J . (2001). The effect of hydrogen peroxide on the cyclin D expression in fibroblasts. Cell Mol Life Sci 58: 990–996.
Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG et al. (1969). The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun 36: 891–897.
Masui Y, Markert CL . (1971). Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145.
Mauro F, Grasso A, Tolmach LJ . (1969). Variations in sulfhydryl, disulfide, and protein content during synchronous and asynchronous growth of HeLa cells. Biophys J 9: 1377–1397.
McCord JM, Fridovich I . (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055.
Menendez S, Khan Z, Coomber DW, Lane DP, Higgins M, Koufali MM et al. (2003). Oligomerization of the human ARF tumor suppressor and its response to oxidative stress. J Biol Chem 278: 18720–18729.
Menon SG, Coleman MC, Walsh SA, Spitz DR, Goswami PC . (2005). Differential susceptibility of nonmalignant human breast epithelial cells and breast cancer cells to thiol antioxidant-induced G(1)-delay. Antioxid Redox Signal 7: 711–718.
Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H et al. (2003). Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res 63: 2109–2117.
Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T . (2003). Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem 278: 50226–50233.
Nevins JR . (1992). E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 258: 424–429.
Oberley TD, Oberley LW, Slattery AF, Lauchner LJ, Elwell JH . (1990). Immunohistochemical localization of antioxidant enzymes in adult Syrian hamster tissues and during kidney development. Am J Pathol 137: 199–214.
Oberley TD, Schultz JL, Li N, Oberley LW . (1995). Antioxidant enzyme levels as a function of growth state in cell culture. Free Radical Biol Med 19: 53–65.
Obin M, Shang F, Gong X, Handelman G, Blumberg J, Taylor A . (1998). Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB J 12: 561–569.
Ohba M, Shibanuma M, Kuroki T, Nose K . (1994). Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol 126: 1079–1088.
Okuyama H, Shimahara Y, Kawada N, Seki S, Kristensen DB, Yoshizato K et al. (2001). Regulation of cell growth by redox-mediated extracellular proteolysis of platelet-derived growth factor receptor beta. J Biol Chem 276: 28274–28280.
Pan W, Cox S, Hoess RH, Grafstrom RH . (2001). A cyclin D1/cyclin-dependent kinase 4 binding site within the C domain of the retinoblastoma protein. Cancer Res 61: 2885–2891.
Pani G, Colavitti R, Bedogni B, Anzevino R, Borrello S, Galeotti T . (2000). A redox signaling mechanism for density-dependent inhibition of cell growth. J Biol Chem 275: 38891–38899.
Pardee AB . (1974). A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA 71: 1286–1290.
Pines J, Hunter T . (1989). Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58: 833–846.
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B . (1997). A model for p53-induced apoptosis. Nature 389: 300–305.
Rainwater R, Parks D, Anderson ME, Tegtmeyer P, Mann K . (1995). Role of cysteine residues in regulation of p53 function. Mol Cell Biol 15: 3892–3903.
Rapkine L . (1931). Su les processus chimiques au cours de la division cellulaire. Ann Physiol Physiochem Biol 7: 382–418.
Rhee SG . (1999). Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31: 53–59.
Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER . (1999). A family of novel peroxidases, peroxiredoxins. Biofactors 10: 207–209.
Sarsour EH, Agarwal M, Pandita TK, Oberley LW, Goswami PC . (2005). Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem 280: 18033–18041.
Savitsky PA, Finkel T . (2002). Redox regulation of Cdc25C. J Biol Chem 277: 20535–20540.
Schafer FQ, Buettner GR . (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol Med 30: 1191–1212.
Schrader M, Fahimi HD . (2004). Mammalian peroxisomes and reactive oxygen species. Histochem Cell Biol 122: 383–393.
Sebastian B, Kakizuka A, Hunter T . (1993). Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci USA 90: 3521–3524.
Sherr CJ . (1995). Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians 107: 181–186.
Stirpe F, Higgins T, Tazzari PL, Rozengurt E . (1991). Stimulation by xanthine oxidase of 3T3 Swiss fibroblasts and human lymphocytes. Exp Cell Res 192: 635–638.
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T . (1995). Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299.
Tan M, Li S, Swaroop M, Guan K, Oberley LW, Sun Y . (1999). Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem 274: 12061–12066.
Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C et al. (2002). E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol cell 9: 1017–1029.
Tanaka T, Nakamura H, Nishiyama A, Hosoi F, Masutani H, Wada H et al. (2000). Redox regulation by thioredoxin superfamily; protection against oxidative stress and aging. Free Radical Res 33: 851–855.
Temin HM . (1971). Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol 78: 161–170.
Toledano MB, Leonard WJ . (1991). Modulation of transcription factor NF-kappa B binding activity by oxidation–reduction in vitro. Proc Natl Acad Sci USA 88: 4328–4332.
Tu BP, Kudlicki A, Rowicka M, McKnight SL . (2005). Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science 310: 1152–1158.
Wang HP, Schafer FQ, Goswami PC, Oberley LW, Buettner GR . (2003). Phospholipid hydroperoxide glutathione peroxidase induces a delay in G1 of the cell cycle. Free Radical Res 37: 621–630.
Yamamoto K, Volkl A, Hashimoto T, Fahimi HD . (1988). Catalase in guinea pig hepatocytes is localized in cytoplasm, nuclear matrix and peroxisomes. Eur J Cell Biol 46: 129–135.
Yamauchi A, Bloom ET . (1997). Control of cell cycle progression in human natural killer cells through redox regulation of expression and phosphorylation of retinoblastoma gene product protein. Blood 89: 4092–4099.
Zangar RC, Davydov DR, Verma S . (2004). Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199: 316–331.
Zou Y, Ewton DZ, Deng X, Mercer SE, Friedman E . (2004). Mirk/dyrk1B kinase destabilizes cyclin D1 by phosphorylation at threonine 288. J Biol Chem 279: 27790–27798.
Acknowledgements
We thank Ms Kellie Bodeker with editorial assistance. This work was supported by funding from NIH CA 111365 and The University of Iowa Carver Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menon, S., Goswami, P. A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26, 1101–1109 (2007). https://doi.org/10.1038/sj.onc.1209895
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209895
Keywords
This article is cited by
-
A mitotic NADPH upsurge promotes chromosome segregation and tumour progression in aneuploid cancer cells
Nature Metabolism (2023)
-
From amino-acid to disease: the effects of oxidation on actin-myosin interactions in muscle
Journal of Muscle Research and Cell Motility (2023)
-
Metformin in therapeutic applications in human diseases: its mechanism of action and clinical study
Molecular Biomedicine (2022)
-
Quantitative redox proteomics revealed molecular mechanisms of salt tolerance in the roots of sugar beet monomeric addition line M14
Botanical Studies (2022)
-
Finding an efficient tetramethylated hydroxydiethylene of resveratrol analogue for potential anticancer agent
BMC Chemistry (2020)