Abstract
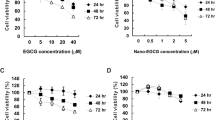
Cigarette smoke is a powerful inducer of inflammatory responses resulting in disruption of major cellular pathways with transcriptional and genomic alterations driving the cells towards carcinogenesis. Cell culture and animal model studies indicate that (−)-epigallocatechin-3-gallate (EGCG), the major polyphenol present in green tea, possesses potent anti-inflammatory and antiproliferative activity capable of selectively inhibiting cell growth and inducing apoptosis in cancer cells without adversely affecting normal cells. Here, we demonstrate that EGCG pretreatment (20–80 μ M) of normal human bronchial epithelial cells (NHBE) resulted in significant inhibition of cigarette smoke condensate (CSC)-induced cell proliferation. Nuclear factor-κB (NF-κB) controls the transcription of genes involved in immune and inflammatory responses. In most cells, NF-κB prevents apoptosis by mediating cell survival signals. Pretreatment of NHBE cells with EGCG suppressed CSC-induced phosphorylation of IκBα, and activation and nuclear translocation of NF-κB/p65. NHBE cells transfected with a luciferase reporter plasmid containing an NF-κB-inducible promoter sequence showed an increased reporter activity after CSC exposure that was specifically inhibited by EGCG pretreatment. Immunoblot analysis showed that pretreatment of NHBE cells with EGCG resulted in a significant downregulation of NF-κB-regulated proteins cyclin D1, MMP-9, IL-8 and iNOS. EGCG pretreatment further inhibited CSC-induced phosphorylation of ERK1/2, JNK and p38 MAPKs and resulted in a decreased expression of PI3K, AKT and mTOR signaling molecules. Taken together, our data indicate that EGCG can suppress NF-κB activation as well as other pro-survival pathways such as PI3K/AKT/mTOR and MAPKs in NHBE cells, which may contribute to its ability to suppress inflammation, proliferation and angiogenesis induced by cigarette smoke.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- EGCG:
-
(−)-epigallocatechin-3-gallate
- NHBE:
-
normal human bronchial epithelial cells
- CSC:
-
cigarette smoke condensate
- NF-κB:
-
nuclear factor-κB
- mTOR:
-
mammalian target of rapamycin
References
Anderson GP, Bozinovski S . (2003). Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci 24: 71–76.
Baeuerle PA, Baltimore D . (1994). Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179.
Coussens LM, Werb Z . (2002). Inflammation and cancer. Nature 420: 860–867 (Review).
Doss MX, Potta SP, Hescheler J, Sachinidis A . (2005). Trapping of growth factors by catechins: a possible therapeutical target for prevention of proliferative diseases. J Nutr Biochem 16: 259–266.
Ferrara N, Gerber HP, LeCouter J . (2003). The biology of VEGF and its receptors. Nat Med 9: 669–676.
Ho YS, Chen CH, Wang YJ, Pestell RG, Albanese C, Chen RJ et al. (2005). Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol Appl Pharmacol 205: 133–148.
Hunninghake GW, Crystal RG . (1983). Cigarette smoking and lung destruction. Accumulation of neutrophils in the lungs of cigarette smokers. Am Rev Respir Dis 128: 833–838.
Jin Z, Gao F, Flagg T, Deng X . (2004). Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem 279: 23837–23844.
Johnson MK, Loo G . (2000). Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat Res 459: 211–218.
Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM et al. (2002). Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res 62: 6850–6856.
Konopka TE, Barker JE, Bamford TL, Guida E, Anderson RL, Stewart AG . (2001). Nitric oxide synthase II gene disruption implications for tumor growth and vascular endothelial growth factor production. Cancer Res 61: 3182–3187.
Kurie JM, Shin HJ, Lee JS, Morice RC, Ro JY, Lippman SM et al. (1996). Increased epidermal growth factor receptor expression in metaplastic bronchial epithelium. Clin Cancer Res 2: 1787–1793.
Kyosseva SV . (2004). Mitogen-activated protein kinase signaling. Int Rev Neurobiol 59: 201–220.
Lin A, Karin M . (2003). NF-kappaB in cancer: a marked target. Semin Cancer Biol 13: 107–114.
Magnani M, Crinelli R, Bianchi M, Antonelli A . (2000). The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-κB (NF-κB). Curr Drug Targets 1: 387–399.
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC . (2002). Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10: 151–162.
Miller DP, De Vivo I, Neuberg D, Wain JC, Lynch TJ, Su L et al. (2003). Association between self-reported environmental tobacco smoke exposure and lung cancer: modification by GSTP1 polymorphism. Int J Cancer 104: 758–763.
Mossman BT, Lounsbury KM, Reddy SP . (2006). Oxidants and signaling by mitogen-activated protein kinases (MAPK) in lung epithelium. Am J Respir Cell Mol Biol 34: 666–669.
Nishikawa M, Kakemizu N, Ito T, Kudo M, Kaneko T, Suzuki M et al. (1999). Superoxide mediates cigarette smoke-induced infiltration of neutrophils into the airways through nuclear factor-kappaB activation and IL-8 mRNA expression in guinea pigs in vivo. Am J Respir Cell Mol Biol 20: 189–198.
Ozlu T, Bulbul Y . (2005). Smoking and lung cancer. Tuberk Toraks 53: 200–209.
Patel JD . (2005). Lung cancer in women. J Clin Oncol 23: 3212–3218.
Petty WJ, Dragnev KH, Dmitrovsky E . (2003). Cyclin D1 as a target for chemoprevention. Lung Cancer 41 (Suppl 1): S155–S161.
Philip M, Rowley DA, Schreiber H . (2004). Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol 14: 433–439.
Rao CV . (2004). Nitric oxide signaling in colon cancer chemoprevention. Mutat Res 555: 107–119.
Rusznak C, Sapsford RJ, Devalia JL, Shah SS, Hewitt EL, Lamont AG et al. (2001). Interaction of cigarette smoke and house dust mite allergens on inflammatory mediator release from primary cultures of human bronchial epithelial cells. Clin Exp Allergy 31: 226–238.
Sahnoun Z, Jamoussi K, Zeghal KM . (1998). Free radicals and antioxidants: physiology, human pathology and therapeutic aspects (part II). Therapie 53: 315–339.
Sasco AJ, Secretan MB, Straif K . (2004). Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer 45 (Suppl 2): S3–S9.
Shiraga M, Yano S, Yamamoto A, Ogawa H, Goto H, Miki T et al. (2002). Organ heterogeneity of host-derived matrix metalloproteinase expression and its involvement in multiple-organ metastasis by lung cancer cell lines. Cancer Res 62: 5967–5973.
Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR . (2002). Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem 277: 3863–3869.
Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF et al. (2001). Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell Mol Physiol 280: L165–L172.
Tsao AS, McDonnell T, Lam S, Putnam JB, Bekele N, Hong WK et al. (2003). Increased phospho-AKT Ser(473) expression in bronchial dysplasia: implications for lung cancer prevention studies. Cancer Epidemiol Biomarkers Prev 12: 660–664.
Tsurutani J, Castillo SS, Brognard J, Granville CA, Zhang C, Gills JJ et al. (2005). Tobacco components stimulate Akt-dependent proliferation and NF-κB-dependent survival in lung cancer cells. Carcinogenesis 26: 1182–1195.
Valen G, Yan ZQ, Hansson GKJ . (2001). Nuclear factor kappa-B and the heart. Am Coll Cardiol 38: 307–314.
Vihinen P, Kahari VM . (2002). Matrix metalloproteinases in cancer: prognostic markers and therapeutic Targets. Int J Cancer 99: 157–166.
Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A et al. (2006). Differential protease, innate immunity and NF{kappa}B induction profiles during lung inflammation induced by sub-chronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol 290: L931–L945.
West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM et al. (2003). Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81–90.
Wislez M, Spencer ML, Izzo JG, Juroske DM, Balhara K, Cody DD et al. (2005). Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res 65: 3226–3335.
Wistuba II, Mao L, Gazdar AF . (2002). Smoking molecular damage in bronchial epithelium. Oncogene 2: 7298–7306.
Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG . (2002). The role of nitric oxide in cancer. Cell Res 12: 311–320.
Yamamoto T, Hsu S, Lewis J, Wataha J, Dickinson D, Singh B et al. (2003). Green tea polyphenol causes differential oxidative environments in tumor versus normal epithelial cells. Pharmacol Exp Ther 307: 230–236.
Yang CS, Liao J, Yang GY, Lu G . (2005). Inhibition of lung tumorigenesis by tea. Exp Lung Res 31: 135–144.
Zhang Q, Adiseshaiah P, Reddy SP . (2005). Matrix metalloproteinase/epidermal growth factor receptor/mitogen-activated protein kinase signaling regulates fra-1 induction by cigarette smoke in lung epithelial cells. Am J Respir Cell Mol Biol 32: 72–81.
Zhong CY, Zhou YM, Douglas GC, Witschi H, Pinkerton KE . (2005). MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis 26: 2187–21895.
Acknowledgements
This study was supported by the developmental funds from US Public Health Service Grant 5P30 CA 14520 and also used resources of USPHS grants R01 CA 78809 and R01 CA 101039.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Syed, D., Afaq, F., Kweon, MH. et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-κB activation in normal human bronchial epithelial cells. Oncogene 26, 673–682 (2007). https://doi.org/10.1038/sj.onc.1209829
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209829
Keywords
This article is cited by
-
NPS2143 Inhibits MUC5AC and Proinflammatory Mediators in Cigarette Smoke Extract (CSE)-Stimulated Human Airway Epithelial Cells
Inflammation (2017)
-
(-)-Epigallocatechin-3-gallate inhibits nasopharyngeal cancer stem cell self-renewal and migration and reverses the epithelial–mesenchymal transition via NF-κB p65 inactivation
Tumor Biology (2015)
-
Dietary phytochemicals alter epigenetic events and signaling pathways for inhibition of metastasis cascade
Cancer and Metastasis Reviews (2014)
-
(−)-Epigallocatechin-3-gallate inhibits human papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in non-small cell lung cancer cells by targeting HIF-1α
Cancer Chemotherapy and Pharmacology (2013)
-
Apple extracts attenuate tumor necrosis factor-α-induced nuclear factor-κB activation by inhibiting IκB kinase and proteasome in A549 human non-small lung carcinoma cells
Food Science and Biotechnology (2012)