Abstract
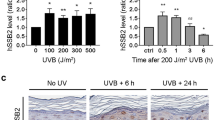
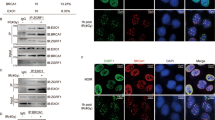
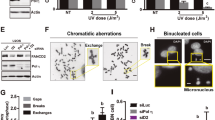
In response to DNA damage, mammalian cells activate various DNA repair pathways to remove DNA lesions and, meanwhile, halt cell cycle progressions to allow sufficient time for repair. The nucleotide excision repair (NER) and the ATR-dependent cell cycle checkpoint activation are two major cellular responses to DNA damage induced by UV irradiation. However, how these two processes are coordinated in the response is poorly understood. Here we showed that the essential NER factor XPA (xeroderma pigmentosum group A) underwent nuclear accumulation upon UV irradiation, and strikingly, such an event occurred in an ATR (Ataxia-Telangiectasia mutated and RAD3-related)-dependent manner. Either treatment of cells with ATR kinase inhibitors or transfection of cells with small interfering RNA targeting ATR compromised the UV-induced XPA nuclear translocation. Consistently, the ATR-deficient cells displayed no substantial XPA nuclear translocation while the translocation remained intact in ATM (Ataxia-Telangiectasia mutated)-deficient cells in response to UV irradiation. Moreover, we found that ATR is required for the UV-induced nuclear focus formation of XPA. Taken together, our results suggested that the ATR checkpoint pathway may modulate NER activity through the regulation of XPA redistribution in human cells upon UV irradiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abraham RT . (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196.
Adimoolam S, Ford JM . (2002). p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc Natl Acad Sci USA 99: 12985–12990.
Barr SM, Leung CG, Chang EE, Cimprich KA . (2003). ATR kinase activity regulates the intranuclear translocation of ATR and RPA following ionizing radiation. Curr Biol 13: 1047–1051.
Bartek J, Lukas C, Lukas J . (2004). Checking on DNA damage in S-phase. Nat Rev Mol Cell Biol 5: 792–804.
Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL et al. (1998). Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J 17: 159–169.
Costa RM, Chigancas V, Galhardo Rda S, Carvalho H, Menck CF . (2003). The eukaryotic nucleotide excision repair pathway. Biochimie 85: 1083–1099.
Dart DA, Adams KE, Akerman I, Lakin ND . (2004). Recruitment of the cell cycle checkpoint kinase ATR to chromatin during S-phase. J Biol Chem 279: 16433–16440.
Feng Z, Kachnic L, Zhang J, Powell SN, Xia F . (2004). DNA damage induces p53-dependent BRCA1 nuclear export. J Biol Chem 279: 28574–28584.
Ford JM . (2005). Regulation of DNA damage recognition and nucleotide excision repair: another role for p53. Mutat Res 577: 195–202.
Garinis GA, Mitchell JR, Moorhouse MJ, Hanada K, de Waard H, Vandeputte D et al. (2005). Transcriptome analysis reveals cyclobutane pyrimidine dimers as a major source of UV-induced DNA breaks. EMBO J 24: 3952–3962.
Gately DP, Hittle JC, Chan GK, Yen TJ . (1998). Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol Biol Cell 9: 2361–2374.
Giannattasio M, Lazzaro F, Longhese MP, Plevani P, Muzi-Falconi M . (2004). Physical and functional interactions between nucleotide excision repair and DNA damage checkpoint. EMBO J 23: 429–438.
Guzder SN, Sommers CH, Prakash L, Prakash S . (2006). Complex formation with damage recognition protein Rad14 is essential for Saccharomyces cerevisiae Rad1–Rad10 nuclease to perform its function in nucleotide excision repair in vivo. Mol Cell Biol 26: 1135–1141.
Iakoucheva LM, Kimzey AL, Masselon CD, Smith RD, Dunker AK, Ackerman EJ . (2001). Aberrant mobility phenomena of the DNA repair protein XPA. Protein Sci 10: 1353–1362.
Jiang H, Yang LY . (1999). Cell cycle checkpoint abrogator UCN-01 inhibits DNA repair: association with attenuation of the interaction of XPA and ERCC1 nucleotide excision repair proteins. Cancer Res 59: 4529–4534.
Kastan MB, Bartek J . (2004). Cell-cycle checkpoints and cancer. Nature 432: 316–323.
Kastan MB, Lim DS . (2000). The many substrates and functions of ATM. Nat Rev Mol Cell Biol 1: 179–186.
Kim ST, Lim DS, Canman CE, Kastan MB . (1999). Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem 274: 37538–37543.
Koberle B, Roginskaya V, Wood RD . (2006). XPA protein as a limiting factor for nucleotide excision repair and UV sensitivity in human cells. DNA Repair (Amst) 5: 641–648.
Liu Y, Liu Y, Yang Z, Utzat C, Wang G, Basu AK et al. (2005). Cooperative interaction of human XPA stabilizes and enhances specific binding of XPA to DNA damage. Biochemistry 44: 7361–7368.
Miura N, Miyamoto I, Asahina H, Satokata I, Tanaka K, Okada Y . (1991). Identification and characterization of xpac protein, the gene product of the human XPAC (xeroderma pigmentosum group A complementing) gene. J Biol Chem 266: 19786–19789.
Miyamoto I, Miura N, Niwa H, Miyazaki J, Tanaka K . (1992). Mutational analysis of the structure and function of the xeroderma pigmentosum group A complementing protein. Identification of essential domains for nuclear localization and DNA excision repair. J Biol Chem 267: 12182–12187.
Neecke H, Lucchini G, Longhese MP . (1999). Cell cycle progression in the presence of irreparable DNA damage is controlled by a Mec1- and Rad53-dependent checkpoint in budding yeast. EMBO J 18: 4485–4497.
Nghiem P, Park PK, Kim Ys YS, Desai BN, Schreiber SL . (2002). ATR is not required for p53 activation but synergizes with p53 in the replication checkpoint. J Biol Chem 277: 4428–4434.
Nitta M, Saijo M, Kodo N, Matsuda T, Nakatsu Y, Tamai H et al. (2000). A novel cytoplasmic GTPase XAB1 interacts with DNA repair protein XPA. Nucleic Acids Res 28: 4212–4218.
O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA . (2003). A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33: 497–501.
O'Neill T, Dwyer AJ, Ziv Y, Chan DW, Lees-Miller SP, Abraham RH et al. (2000). Utilization of oriented peptide libraries to identify substrate motifs selected by ATM. J Biol Chem 275: 22719–22727.
Rademakers S, Volker M, Hoogstraten D, Nigg AL, Mone MJ, Van Zeeland AA et al. (2003). Xeroderma pigmentosum group A protein loads as a separate factor onto DNA lesions. Mol Cell Biol 23: 5755–5767.
Riedl T, Hanaoka F, Egly JM . (2003). The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J 22: 5293–5303.
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S . (2004). Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73: 39–85.
Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM et al. (1999). Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res 59: 4375–4382.
Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT . (1998). Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res 58: 4375–4382.
Thoma BS, Vasquez KM . (2003). Critical DNA damage recognition functions of XPC-hHR23B and XPA-RPA in nucleotide excision repair. Mol Carcinogen 38: 1–13.
Volker M, Mone MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W et al. (2001). Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell 8: 213–224.
Wang J, Chin MY, Li G . (2006). The novel tumor suppressor p33ING2 enhances nucleotide excision repair via inducement of histone H4 acetylation and chromatin relaxation. Cancer Res 66: 1906–1911.
Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF et al. (1998). Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proc Natl Acad Sci USA 95: 7445–7450.
Wu X, Shell SM, Yang Z, Zou Y . (2006). Phosphorylation of nucleotide excision repair factor XPA by ATR-dependent checkpoint pathway promotes cell survival in response to UV irradiation. Cancer Res 66: 2997–3005.
Wu X, Shell SM, Zou Y . (2005a). Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene 24: 4728–4735.
Wu Y, Lu Y, Hu Y, Li R . (2005b). Cyclic AMP-dependent modification of gonad-selective TAF(II)105 in a human ovarian granulosa cell line. J Cell Biochem 96: 751–759.
Xu B, Kim S, Kastan MB . (2001). Involvement of Brca1 in S-phase and G(2)-phase checkpoints after ionizing irradiation. Mol Cell Biol 21: 3445–3450.
Xu B, Kim ST, Lim DS, Kastan MB . (2002). Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol 22: 1049–1059.
Yu S, Teng Y, Lowndes NF, Waters R . (2001). RAD9, RAD24, RAD16 and RAD26 are required for the inducible nucleotide excision repair of UV-induced cyclobutane pyrimidine dimers from the transcribed and non-transcribed regions of the Saccharomyces cerevisiae MFA2 gene. Mutat Res 485: 229–236.
Zhou BB, Elledge SJ . (2000). The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439.
Zou L, Cortez D, Elledge SJ . (2002). Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev 16: 198–208.
Acknowledgements
We thank Dr Priscilla B Wyrick for her generous assistance in immunofluorescence measurements. This study was supported by NCI Grant CA86927 (to YZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Shell, S., Liu, Y. et al. ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation. Oncogene 26, 757–764 (2007). https://doi.org/10.1038/sj.onc.1209828
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209828
Keywords
This article is cited by
-
RETRACTED ARTICLE: The melanocortin signaling cAMP axis accelerates repair and reduces mutagenesis of platinum-induced DNA damage
Scientific Reports (2017)
-
Orchestral maneuvers at the damaged sites in nucleotide excision repair
Cellular and Molecular Life Sciences (2015)
-
Coordinated regulation of XPA stability by ATR and HERC2 during nucleotide excision repair
Oncogene (2014)
-
Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress
Cellular and Molecular Life Sciences (2014)