Abstract
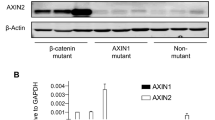
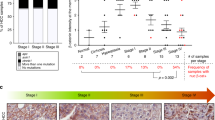
Perturbations to the Wnt signaling pathway have been implicated in a large proportion of human hepatocellular carcinomas (HCCs). Activating β-catenin mutations and loss of function mutations in Axin1 are thought to be functionally equivalent. We examined the Wnt pathway in HCC by comparing the expression of β-catenin target genes and the level of β-catenin-dependent transcriptional activation, in 45 HCC tumors and four cell lines. Among these samples, β-catenin and AXIN1 were mutated in 20 and seven cases, respectively. We found a significant correlation between activated β-catenin mutations and overexpression of mRNA for the target genes glutamine synthetase (GS), G-protein-coupled receptor (GPR)49 and glutamate transporter (GLT)-1 (P=0.0001), but not for the genes ornithine aminotransferase, LECT2, c-myc and cyclin D1. We also showed that GS is a good immunohistochemical marker of β-catenin activation in HCC. However, we observed no induction of GS, GPR49 or GLT-1 in the five inactivated Axin1 tumors. β-Catenin-dependent transcriptional activation in two Axin1-mutated HCC cell lines was much weaker than in β-catenin-mutated cell lines. Our results strongly suggest that in HCC, contrary to expectation, the loss of function of Axin1 is not equivalent to the gain of function of β-catenin. Our results also suggest that the tumor suppressor function of Axin1 in HCC may be related to another, non-Wnt pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Befeler AS, Di Bisceglie AM . (2002). Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 122: 1609–1619.
Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH et al. (2002). New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 21: 8293–8301.
Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A et al. (2004). Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA 101: 17216–17221.
de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O et al. (1998). Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 95: 8847–8851.
Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y et al. (2001). Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol 21: 5132–5141.
Giles RH, van Es JH, Clevers H . (2003). Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1–24.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT et al. (1998). Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512.
Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY . (2000). Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 157: 763–770.
Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G et al. (1999). Beta-catenin mutations are frequent in human hepatocellular carcinomas. Am J Pathol 155: 1795–1801.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW et al. (1997). Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787.
Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F et al. (2001). Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology 120: 1763–1773.
Laurent-Puig P, Zucman-Rossi J . (2006). Genetics of hepatocellular tumors. Oncogene 25: 3778–3786.
Legoix P, Bluteau O, Bayer J, Perret C, Balabaud C, Belghiti J et al. (1999). Beta-catenin mutations in hepatocellular carcinoma correlate with a low. Oncogene 18: 4044–4046.
Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S et al. (1998). Activation of the beta-catenin gene in primary hepatocellular. Cancer Res 58: 2524–2527.
Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA . (1999). Nuclear accumulation of mutated beta-catenin in hepatocellular. Am J Pathol 155: 703–710.
Ovejero C, Cavard C, Perianin A, Hakvoort T, Vermeulen J, Godard C et al. (2004). Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology 40: 167–176.
Rui Y, Xu Z, Lin S, Li Q, Rui H, Luo W et al. (2004). Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J 23: 4583–4594.
Salahshor S, Woodgett JR . (2005). The links between axin and carcinogenesis. J Clin Pathol 58: 225–236.
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T et al. (2000). AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 24: 245–250.
Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM et al. (2002). Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 21: 4863–4871.
Terris B, Pineau P, Bregeaud L, Valla D, Belghiti J, Tiollais P et al. (1999). Close correlation between beta-catenin gene alterations and nuclear. Oncogene 18: 6583–6588.
Tetsu O, McCormick F . (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426.
Ueta T, Ikeguchi M, Hirooka Y, Kaibara N, Terada T . (2002). Beta-catenin and cyclin D1 expression in human hepatocellular carcinoma. Oncol Rep 9: 1197–1203.
Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M et al. (2003). Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology 37: 528–533.
Zhang Y, Neo SY, Han J, Lin SC . (2000). Dimerization choices control the ability of axin and dishevelled to activate c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem 275: 25008–25014.
Zhang Y, Neo SY, Wang X, Han J, Lin SC . (1999). Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem 274: 35247–35254.
Acknowledgements
We thank Valérie Paradis and Jacques Belghiti at Beaujon Hospital (Clichy, France) who provided four AXIN1 tumor samples for validation. We also thank all the member of the C Perret's team for their helpful discussions. This work was supported by INSERM, CNRS, the ‘Comite de Paris de la Ligue Nationale contre le Cancer’, the ‘Association pour la Recherche contre le Cancer’ and the SNFGE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zucman-Rossi, J., Benhamouche, S., Godard, C. et al. Differential effects of inactivated Axin1 and activated β-catenin mutations in human hepatocellular carcinomas. Oncogene 26, 774–780 (2007). https://doi.org/10.1038/sj.onc.1209824
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209824
Keywords
This article is cited by
-
Pathologie des Hepatozellulären Karzinoms
Der Pathologe (2022)
-
Hepatozelluläres Karzinom
Der Pathologe (2020)
-
GREB1 induced by Wnt signaling promotes development of hepatoblastoma by suppressing TGFβ signaling
Nature Communications (2019)
-
WDR34 Activates Wnt/Beta-Catenin Signaling in Hepatocellular Carcinoma
Digestive Diseases and Sciences (2019)
-
Ajuba inhibits hepatocellular carcinoma cell growth via targeting of β-catenin and YAP signaling and is regulated by E3 ligase Hakai through neddylation
Journal of Experimental & Clinical Cancer Research (2018)