Abstract
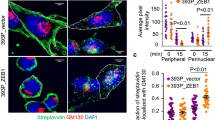
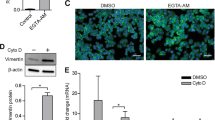
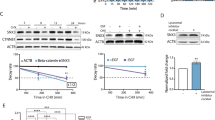
Oncogenic Ras interferes with adhesive functions of epithelial cells, but requires tumor growth factor β (TGFβ) signaling to cause epithelial–mesenchymal transition (EMT) and tumor progression in model systems. To investigate the mechanisms by which Ras and TGFβ pathways cooperate in EMT induction, we introduced a tamoxifen-inducible version of Raf-1 (RafER) into fully polarized, mammary epithelial cells (EpH4). EMT characterized by loss of E-cadherin expression and upregulation of invasiveness-promoting genes was induced by TGFβ plus 4-hydroxytamoxifen (4HT) activation of RafER. Downregulation of E-cadherin by RafER plus TGFβ was detectable in total cell lysates after 48 h and much earlier in detergent-insoluble fractions of E-cadherin. Both pathways cooperated to strongly enhance endocytosis of E-cadherin, mainly via the clathrin-dependent route. Pulse-chase experiments showed decreased E-cadherin protein stability in cells stimulated with TGFβ and 4HT and increased E-cadherin half-life in the presence of monensin. Monensin and chloroquine prevented E-cadherin degradation to different extent, but only monensin effectively blocked the loss of E-cadherin from the junctional complexes. Both lysosome inhibitors caused accumulation of E-cadherin vesicles, some of which were positive for Cathepsin D and lysosome-associated membrane protein 1 (LAMP-1). In addition, TGFβ and mitogen-activated protein kinase hyperactivation synergistically induced E-cadherin ubiquitination, suggesting that the cooperation of Raf and TGFβ favors lysosomal degradation of E-cadherin instead of its recycling. Our data indicate that early stages of EMT involve cooperative, post-translational downregulation of E-cadherin, whereas loss of E-cadherin via transcriptional repression is a late event in EMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Akhtar N, Hotchin NA . (2001). Mol Biol Cell 12: 847–862.
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J et al. (2000). Nat Cell Biol 2: 84–89.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG et al. (2000). Nat Cell Biol 2: 76–83.
Christofori G, Semb H . (1999). Trends Biochem Sci 24: 73–76.
Derynck R, Akhurst RJ, Balmain A . (2002). Nat Genet 29: 117–129.
Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J et al. (2002). Nat Cell Biol 4: 222–231.
Grunert S, Jechlinger M, Beug H . (2003). Nat Rev Mol Cell Biol 4: 657–665.
Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E et al. (2002). J Biol Chem 277: 39209–39216.
Gumbiner BM . (2000). J Cell Biol 148: 399–404.
Harbeck N, Thomssen C, Berger U, Ulm K, Kates RE, Hofler H et al. (1999). Breast Cancer Res Treat 54: 147–157.
Ivanov AI, Nusrat A, Parkos CA . (2004). Mol Biol Cell 15: 176–188 (E-pub ahead of print 2003 Oct 03).
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J et al. (2002a). J Cell Biol 156: 299–313.
Janda E, Litos G, Grunert S, Downward J, Beug H . (2002b). Oncogene 21: 5148–5159.
Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF et al. (1999). Oncogene 18: 6776–6784.
Kemler R . (1992). Semin Cell Biol 3: 149–155.
Le TL, Yap AS, Stow JL . (1999). J Cell Biol 146: 219–232.
Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M et al. (2000). Genes Dev 14: 2610–2622.
Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM et al. (1999). Proc Natl Acad Sci USA 96: 4844–4849.
Lisanti MP, Tang ZL, Sargiacomo M . (1993). J Cell Biol 123: 595–604.
Negroiu G, Dwek RA, Petrescu SM . (2005). Biochem Biophys Res Commun 328: 914–921.
Nose A, Takeichi M . (1986). J Cell Biol 103: 2649–2658.
Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E . (1996). Genes Dev 10: 2462–2477.
Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C . (2005). Mol Cell Biol 25: 389–402.
Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J et al. (1992). Cell 71: 1103–1116.
Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA et al. (2003). J Biol Chem 278: 1372–1379 (E-pub ahead of print 2002 Oct 19).
Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J . (2001). Genes Dev 15: 981–994.
Shen L, Turner JR . (2005). Mol Biol Cell 16: 3919–3936 (E-pub ahead of print 2005 June 15).
Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P et al. (2005). Proc Natl Acad Sci USA 102: 2760–2765 (E-pub ahead of print 2005 Feb 8).
Sun D, Baur S, Hay ED . (2000). Dev Biol 228: 337–349.
Szuts D, Eresh S, Bienz M . (1998). Genes Dev 12: 2022–2035.
Thiery JP . (2002). Nat Rev Cancer 2: 442–454.
Viebahn C . (1995). Acta Anat 154: 79–97.
Wang LH, Rothberg KG, Anderson RG . (1993). J Cell Biol 123: 1107–1117.
Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M . (1997). Mol Cell Biol 17: 5598–5611.
Wu X, Zhao X, Baylor L, Kaushal S, Eisenberg E, Greene LE . (2001). J Cell Biol 155: 291–300 (E-pub ahead of print 2001 Oct 15).
Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S et al. (2001). Proc Natl Acad Sci USA 98: 6686–6691.
Zhang Y, Feng XH, Derynck R . (1998). Nature 394: 909–913.
Zhu W, Leber B, Andrews DW . (2001). EMBO J 20: 5999–6007.
Acknowledgements
We thank Enrico Avvedimento and Lorenzo Chiariotti (University of Naples ‘Federico II’, Italy) for stimulating discussions and valuable suggestions. Heather Bond (University of Catanzaro, Italy) for critical reading of the manuscript. We also thank Simona Polo (European Institute of Oncology, Milan, Italy) for reagents and helpful hints on experiments concerning monoubiquitination Pasquale Spataro (University of Messina) for help with confocal microscopy, and Yasuyuki Fujita (MRC, London, UK) for kind gift of mouse Hakai cDNA. This work was supported by grants from MIUR, Progetto 12, Cluster-04.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Janda, E., Nevolo, M., Lehmann, K. et al. Raf plus TGFβ-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene 25, 7117–7130 (2006). https://doi.org/10.1038/sj.onc.1209701
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209701
Keywords
This article is cited by
-
P2x4 receptor promotes mammary cancer progression by sustaining autophagy and associated mesenchymal transition
Oncogene (2022)
-
Comparative Analysis of microRNAs that Stratify in vitro Mammary stem and Progenitor Activity Reveals Functionality of Human miR-92b-3p
Journal of Mammary Gland Biology and Neoplasia (2022)
-
Cancer drug resistance induced by EMT: novel therapeutic strategies
Archives of Toxicology (2021)
-
A self-sustaining endocytic-based loop promotes breast cancer plasticity leading to aggressiveness and pro-metastatic behavior
Nature Communications (2020)
-
Direct visualization of the extracellular binding structure of E-cadherins in liquid
Scientific Reports (2020)