Abstract
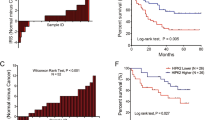
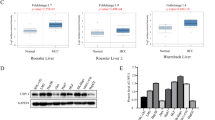
Aberrant activation of the Ras/Raf-1/extracellular-regulated kinase (ERK) pathway has been shown to be involved in the progression of human hepatocellular carcinoma (HCC). However, the mechanism of dysregulation of ERK activation is poorly understood. Recently, we identified Sprouty-related protein with Ena/vasodilator-stimulated phosphoprotein homology-1 domain (Spred) as a physiological inhibitor of the Ras/Raf-1/ERK pathway. In this study, we found that the expression levels of Spred-1 and -2 in human HCC tissue were frequently decreased, comparing with those in adjacent non-tumorous tissue. Moreover, Spred expression levels in HCC tissue were inversely correlated with the incidence of tumor invasion and metastasis. Forced expression of Spred-1 inhibited HCC cell proliferation in vitro and in vivo, which was associated with reduced ERK activation. Spred-1 overexpression also reduced the secretion of matrix metalloproteinase-9 (MMP-9) and MMP-2, which play important roles in tumor invasion and metastasis. In addition, Spred-1 inhibited growth factor-mediated HCC cell motility. These data indicate that the reduction of Spred expression in HCC is one of the causes of the acquisition of malignant features. Thus, Spred could be not only a novel prognostic factor but also a new therapeutic target for human HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Amano M, Fukata Y, Kaibuchi K . (2000). Exp Cell Res 261: 44–51.
Bottazzi ME, Zhu X, Bohmer RM, Assoian RK . (1999). J Cell Biol 146: 1255–1264.
Chen L, Zeng Y, Yang H, Lee TD, French SW, Corrales FJ et al. (2004). FASEB J 18: 914–916.
Coussens LM, Tinkle CL, Hanahan D, Werb Z . (2000). Cell 103: 481–490.
Ehrenreiter K, Piazzolla D, Velamoor V, Sobczak I, Small JV, Takeda J et al. (2005). J Cell Biol 168: 955–964.
Inoue H, Kato R, Fukuyama S, Nonami A, Taniguchi K, Matsumoto K et al. (2005). J Exp Med 201: 73–82.
Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A et al. (1998). Hepatology 27: 951–958.
Johnson GL, Lapadat R . (2002). Science 298: 1911–1912.
Kato R, Nonami A, Taketomi T, Wakioka T, Kuroiwa A, Matsuda Y et al. (2003). Biochem Biophys Res Commun 302: 767–772.
Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E et al. (2001). Hepatology 33: 1087–1097.
Liu JF, Crepin M, Liu JM, Barritault D, Ledoux D . (2002). Biochem Biophys Res Commun 293: 1174–1182.
Marrero JA, Lok AS . (2004). Gastroenterology 127: S113–S119.
Marshall CJ . (1995). Cell 80: 179–185.
Masson V, de la Ballina LR, Munaut C, Wielockx B, Jost M, Maillard C et al. (2005). FASEB J 19: 234–236.
McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF . (2003). Mol Cell Biol 23: 216–228.
Mikula M, Schreiber M, Husak Z, Kucerova L, Ruth J, Wieser R et al. (2001). EMBO J 20: 1952–1962.
Miyoshi K, Wakioka T, Nishinakamura H, Kamio M, Yang L, Inoue M et al. (2004). Oncogene 23: 5567–5576.
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM . (2000). J Clin Oncol 18: 1135–1149.
Nonami A, Kato R, Taniguchi K, Yoshiga D, Taketomi T, Fukuyama S et al. (2004). J Biol Chem 279: 52543–52551.
Potempa S, Ridley AJ . (1998). Mol Biol Cell 9: 2185–2200.
Prehoda KE, Lee DJ, Lim WA . (1999). Cell 97: 471–480.
Ridley AJ, Hall A . (1992). Cell 70: 389–399.
Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A . (1992). Cell 70: 401–410.
Sasaki A, Taketomi T, Kato R, Saeki K, Nonami A, Sasaki M et al. (2003). Nat Cell Biol 5: 427–432.
Schmidt CM, McKillop IH, Cahill PA, Sitzmann JV . (1997). Biochem Biophys Res Commun 236: 54–58.
Stahl S, Ittrich C, Marx-Stoelting P, Kohle C, Altug-Teber O, Riess O et al. (2005). Hepatology 42: 353–361.
Tsuboi Y, Ichida T, Sugitani S, Genda T, Inayoshi J, Takamura M et al. (2004). Liver Int 24: 432–436.
Vaudry D, Stork PJ, Lazarovici P, Eiden LE . (2002). Science 296: 1648–1649.
Volkman BF, Prehoda KE, Scott JA, Peterson FC, Lim WA . (2002). Cell 111: 565–576.
Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K et al. (2001). Nature 412: 647–651.
Yano H, Maruiwa M, Murakami T, Fukuda K, Ito Y, Sugihara S et al. (1988). Acta Pathol Jpn 38: 953–966.
Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y et al. (2004). J Exp Med 199: 1701–1707.
Zaragoza C, Soria E, Lopez E, Browning D, Balbin M, Lopez-Otin C et al. (2002). Mol Pharmacol 62: 927–935.
Zhang D, Bar-Eli M, Meloche S, Brodt P . (2004). J Biol Chem 279: 19683–19690.
Acknowledgements
We thank Ms Masako Sinkawa for her excellent technical assistance and Ms Mieko Hatae and Ms Motoko Gotoh for manuscript preparation. This work was supported by the 21st Century COE Program for Medical Science of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, T., Hisamoto, T., Akiba, J. et al. Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene 25, 6056–6066 (2006). https://doi.org/10.1038/sj.onc.1209635
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209635
Keywords
This article is cited by
-
Bioinformatics analysis for the identification of Sprouty-related EVH1 domain-containing protein 3 expression and its clinical significance in thyroid carcinoma
Scientific Reports (2024)
-
Elevated FBXL6 activates both wild-type KRAS and mutant KRASG12D and drives HCC tumorigenesis via the ERK/mTOR/PRELID2/ROS axis in mice
Military Medical Research (2023)
-
SPOCK2 and SPRED1 function downstream of EZH2 to impede the malignant progression of lung adenocarcinoma in vitro and in vivo
Human Cell (2023)
-
AK2 is an AMP-sensing negative regulator of BRAF in tumorigenesis
Cell Death & Disease (2022)
-
In silico, in vitro screening of antioxidant and anticancer potentials of bioactive secondary metabolites from an endophytic fungus (Curvularia sp.) from Phyllanthus niruri L
Environmental Science and Pollution Research (2022)