Abstract
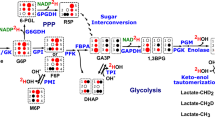
A key hallmark of many cancers, particularly the most aggressive, is the capacity to metabolize glucose at an elevated rate, a phenotype detected clinically using positron emission tomography (PET). This phenotype provides cancer cells, including those that participate in metastasis, a distinct competitive edge over normal cells. Specifically, after rapid entry of glucose into cancer cells on the glucose transporter, the highly glycolytic phenotype is supported by hexokinase (primarily HK II) that is overexpressed and bound to the outer mitochondrial membrane via the porin-like protein voltage-dependent anion channel (VDAC). This protein and the adenine nucleotide transporter move ATP, newly synthesized by the inner membrane located ATP synthase, to active sites on HK II. The abundant amounts of HK II bind both the ATP and the incoming glucose producing the product glucose-6-phosphate, also at an elevated rate. This critical metabolite then serves both as a biosynthetic precursor to support cell proliferation and as a precursor for lactic acid, the latter exiting cancer cells causing an unfavorable environment for normal cells. Although helping facilitate this chemical warfare, HK II via its mitochondrial location also suppresses the death of cancer cells, thus increasing their possibility for metastasis and the ultimate death of the human host. For these reasons, targeting this key enzyme is currently being investigated in several laboratories in a strategy to develop novel therapies that may turn the tide on the continuing struggle to find effective cures for cancer. One such candidate is 3-bromopyruvate that has been shown recently to eradicate advanced stage, PET positive hepatocellular carcinomas in an animal model without apparent harm to the animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ardehali H, Yano Y, Printz RL, Koch S, Whitesell RR, May JM et al. (1996). J Biol Chem 271: 1849–1852.
Arora KK, Parry DM, Pedersen PL . (1992). J Bioenerg Biomembr 24: 47–53.
Arora KK, Pedersen PL . (1988). J Biol Chem 263: 17422–17428.
Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V . (2004). Biochem J 377: 347–355.
Bay DC, Court DA . (2002). Biochem Cell Biol 80: 551–562.
Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J et al. (1993). J Biol Chem 268: 1835–1841.
Bustamante E, Morris HP, Pedersen PL . (1981). J Biol Chem 256: 8699–8704.
Bustamante E, Pedersen PL . (1977). Proc Natl Acad Sci USA 74: 3735–3739.
Bustamante E, Pediaditakis P, He L, Lemasters JJ . (2005). Biochem Biophys Res Commun 334: 907–910.
Campanella ME, Chu H, Low PS . (2005). Proc Natl Acad Sci USA 102: 2402–2407.
Capano M, Crompton M . (2002). Biochem J 367: 169–178.
Cesar MC, Wilson JE . (1998). Arch Biochem Biophys 350: 109–117.
Cesar MC, Wilson JE . (2004). Arch Biochem Biophys 422: 191–196.
Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL . (2004). J Biol Chem 279: 31761–31768.
Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ . (2003). Science 301: 513–517.
Chevrollier A, Loiseau D, Chabi B, Renier G, Douay O, Malthiery Y et al. (2005). J Bioenerg Biomembr 37: 307–317.
Colombini M . (2004). Mol Cell Biochem 256–257: 107–115.
Crompton M . (1999). Biochem J 341 (Part 2): 233–249.
Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM et al. (2003). Nature 424: 952–956.
Don AS, Hogg PJ . (2004). Trends Mol Med 10: 372–378.
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR et al. (2004). Cancer Res 64: 3892–3899.
Fanciulli M, Valentini A, Bruno T, Citro G, Zupi G, Floridi A . (1996). Oncol Res 8: 111–120.
Felgner PL, Messer JL, Wilson JE . (1979). J Biol Chem 254: 4946–4949.
Floridi A, Bruno T, Miccadei S, Fanciulli M, Federico A, Paggi MG . (1998). Biochem Pharmacol 56: 841–849.
Gauthier T, Denis-Pouxviel C, Murat JC . (1990). Int J Biochem 22: 419–423.
Geschwind JF, Georgiades CS, Ko YH, Pedersen PL . (2004). Expert Rev Anticancer Ther 4: 449–457.
Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL . (2002). Cancer Res 62: 3909–3913.
Goel A, Mathupala SP, Pedersen PL . (2003). J Biol Chem 278: 15333–15340.
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N . (2001). Genes Dev 15: 1406–1418.
Graham JF, Cummins CJ, Smith BH, Kornblith PL . (1985). Neurosurgery 17: 537–542.
Granville DJ, Gottlieb RA . (2003). Curr Med Chem 10: 1527–1533.
Johansson T, Berrez JM, Nelson BD . (1985). Biochem Biophys Res Commun 133: 608–613.
Ko YH, Delannoy M, Hullihen J, Chiu W, Pedersen PL . (2003). J Biol Chem 278: 12305–12309.
Ko YH, Pedersen PL, Geschwind JF . (2001). Cancer Lett 173: 83–91.
Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS et al. (2004). Biochem Biophys Res Commun 324: 269–275.
Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP et al. (2004). Nature 427: 461–465.
Kropp ES, Wilson JE . (1970). Biochem Biophys Res Commun 38: 74–79.
Lee MG, Pedersen PL . (2003). J Biol Chem 278: 41047–41058.
Lemasters JJ, Holmuhamedov E . (2006). Biochim Biophys Acta 1762: 181–190.
Macheda ML, Rogers S, Best JD . (2005). J Cell Physiol 202: 654–662.
Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K et al. (2004a). Mol Cell 16: 819–830.
Majewski N, Nogueira V, Robey RB, Hay N . (2004b). Mol Cell Biol 24: 730–740.
Mathupala SP, Heese C, Pedersen PL . (1997a). J Biol Chem 272: 22776–22780.
Mathupala SP, Rempel A, Pedersen PL . (1997b). J Bioenerg Biomembr 29: 339–343.
Mathupala SP, Rempel A, Pedersen PL . (1995). J Biol Chem 270: 16918–16925.
Mathupala SP, Rempel A, Pedersen PL . (2001). J Biol Chem 276: 43407–43412.
Mayer D, Klimek F, Rempel A, Bannasch P . (1997). Biochem Soc Trans 25: 122–127.
Miccoli L, Oudard S, Sureau F, Poirson F, Dutrillaux B, Poupon MF . (1996). Biochem J 313 (Part 3): 957–962.
Nakashima RA, Mangan PS, Colombini M, Pedersen PL . (1986). Biochemistry 25: 1015–1021.
Pastorino JG, Hoek JB . (2003). Curr Med Chem 10: 1535–1551.
Pastorino JG, Shulga N, Hoek JB . (2002). J Biol Chem 277: 7610–7618.
Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Maziere B . (1998). Nucl Med Biol 25: 317–322.
Pedersen PL . (1978). Prog Exp Tumor Res 22: 190–274.
Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH . (2002). Biochim Biophys Acta 1555: 14–20.
Penso J, Beitner R . (1998). Eur J Pharmacol 342: 113–117.
Plas DR, Thompson CB . (2005). Oncogene 24: 7435–7442.
Printz RL, Osawa H, Ardehali H, Koch S, Granner DK . (1997). Biochem Soc Trans 25: 107–112.
Rempel A, Bannasch P, Mayer D . (1994a). Biochem J 303 (Part 1): 269–274.
Rempel A, Bannasch P, Mayer D . (1994b). Biochim Biophys Acta 1219: 660–668.
Rempel A, Mathupala SP, Griffin CA, Hawkins AL, Pedersen PL . (1996a). Cancer Res 56: 2468–2471.
Rempel A, Mathupala SP, Pedersen PL . (1996b). FEBS Lett 385: 233–237.
Rose IA, Warms JV . (1967). J Biol Chem 242: 1635–1645.
Rose IA, Warms JV . (1982). Arch Biochem Biophys 213: 625–634.
Shimizu S, Konishi A, Kodama T, Tsujimoto Y . (2000a). Proc Natl Acad Sci USA 97: 3100–3105.
Shimizu S, Narita M, Tsujimoto Y . (1999). Nature 399: 483–487.
Shimizu S, Shinohara Y, Tsujimoto Y . (2000b). Oncogene 19: 4309–4318.
Shinohara Y, Ishida T, Hino M, Yamazaki N, Baba Y, Terada H . (2000). Eur J Biochem 267: 6067–6073.
Shinohara Y, Sagawa I, Ichihara J, Yamamoto K, Terao K, Terada H . (1997). Biochim Biophys Acta 1319: 319–330.
Smith TA . (1999). Br J Biomed Sci 56: 285–292.
Smith TA . (2000). Br J Biomed Sci 57: 170–178.
Sui D, Wilson JE . (1997). Arch Biochem Biophys 345: 111–125.
Sui D, Wilson JE . (2004). Biochem Biophys Res Commun 319: 768–773.
Tedeschi H, Kinnally KW, Mannella CA . (1989). J Bioenerg Biomembr 21: 451–459.
Tsai HJ, Wilson JE . (1995). Arch Biochem Biophys 316: 206–214.
Tsai HJ, Wilson JE . (1996). Arch Biochem Biophys 329: 17–23.
Tsai HJ, Wilson JE . (1997). Arch Biochem Biophys 338: 183–192.
Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB . (1999). Mol Cell 3: 159–167.
Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M . (2001). J Biol Chem 276: 19414–19419.
Vischer U, Blondel B, Wollheim CB, Hoppner W, Seitz HJ, Iynedjian PB . (1987). Biochem J 241: 249–255.
Vyssokikh M, Brdiczka D . (2004). Mol Cell Biochem 256–257: 117–126.
Vyssokikh MY, Brdiczka D . (2003). Acta Biochim Pol 50: 389–404.
Vyssokikh MY, Zorova L, Zorov D, Heimlich G, Jurgensmeier JJ, Brdiczka D . (2002). Mol Biol Rep 29: 93–96.
Warburg O, Dickens F, Kaiser Wilhelm-Institut für Biologie B. (1930). The Metabolism of Tumours: Investigations from the Kaiser-Wilhelm Institute for Biology, Berlin-Dahlem. Constable: London.
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M et al. (2000). Genes Dev 14: 2060–2071.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al. (2001). Science 292: 727–730.
Wilson JE . (1995). Rev Physiol Biochem Pharmacol 126: 65–198.
Wilson JE . (1997). Biochem Soc Trans 25: 103–107.
Wilson JE . (2003). J Exp Biol 206: 2049–2057.
Wilson JE, Messer JL, Felgner PL . (1983). Methods Enzymol 97: 469–475.
Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V . (2005). Cell Death Differ 12: 751–760.
Zhou H, Hou Q, Chai Y, Hsu YT . (2005). Exp Cell Res 309: 316–328.
Acknowledgements
SPM is supported by Grant IRG-85-003-14 from the American Cancer Society and a grant from the LEARN Foundation, Michigan, YHK by Grant BCTR0402523 from the Susan Komen Breast Cancer Foundation, and PLP by NIH Grants R01CA08018 and R01CA010951.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathupala, S., Ko, Y. & Pedersen, P. Hexokinase II: Cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25, 4777–4786 (2006). https://doi.org/10.1038/sj.onc.1209603
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209603
Keywords
This article is cited by
-
Single-cell low-pass whole genome sequencing accurately detects circulating tumor cells for liquid biopsy-based multi-cancer diagnosis
npj Precision Oncology (2024)
-
ALKBH5 facilitates the progression of infantile hemangioma by increasing FOXF1 expression in a m6A-YTHDF2 dependent manner to activate HK-2 signaling
Molecular and Cellular Biochemistry (2024)
-
PDLIM1 interacts with HK2 to promote gastric cancer progression through enhancing the Warburg effect via Wnt/β-catenin signaling
Cell and Tissue Research (2024)
-
Regulation of tumor metabolism by post translational modifications on metabolic enzymes
Cancer Gene Therapy (2023)
-
Apoptotic proteins with non-apoptotic activity: expression and function in cancer
Apoptosis (2023)