Abstract
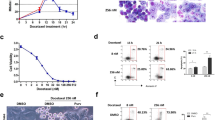
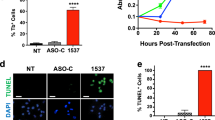
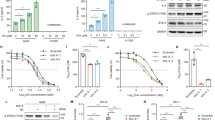
hTERT is the catalytic subunit of the telomerase and is hence required for telomerase maintenance activity and cancer cell immortalization. Here, we show that acute hTERT depletion has no adverse effects on the viability or proliferation of cervical and colon carcinoma cell lines, as evaluated within 72 h after transfection with hTERT-specific small interfering RNAs (siRNAs). Within the same time frame, hTERT depletion facilitated the induction of apoptotic cell death by cisplatin, etoposide, mitomycin C and reactive oxygen species, yet failed to sensitize cells to death induction via the CD95 death receptor. Experiments performed with p53 knockout cells or chemical p53 inhibitors revealed that p53 was not involved in the chemosensitizing effect of hTERT knockdown. However, the proapoptotic Bcl-2 family protein Bax was involved in cell death induction by hTERT siRNAs. Depletion of hTERT facilitated the conformational activation of Bax induced by genotoxic agents. Moreover, Bax knockout abolished the chemosensitizing effect of hTERT siRNAs. Inhibition of mitochondrial membrane permeabilization by overexpression of Bcl-2 or expression of the cytomegalovirus-encoded protein vMIA (viral mitochondrial inhibitor of apoptosis), which acts as a specific Bax inhibitor, prevented the induction of cell death by the combination of hTERT depletion and chemotherapeutic agents. Altogether, our data indicate that hTERT inhibition may constitute a promising strategy for facilitating the induction of the mitochondrial pathway of apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Abbreviations
- ΔΨm:
-
mitochondrial transmembrane potential
- DAPI:
-
4′,6-diaminidino-2-phenylindole
- DiOC6(3):
-
3,3′ dihexyloxacarbocyanine iodide
- FITC:
-
fluorescein isothiocyanate
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- MMP:
-
mitochondrial membrane permeabilization
- PI:
-
propidium iodide
- PS:
-
phosphatidylserine
- siRNA:
-
small interfering RNA
- Z-VAD.fmk:
-
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
References
Belzacq AS, El Hamel C, Vieira HLA, Cohen I, Haouzi D, Metivier D et al. (2001). Oncogene 20: 7579–7587.
Blackburn EH . (2001). Cell 106: 661–673.
Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L et al. (1999). J Clin Invest 104: 263–269.
Cao Y, Li H, Deb S, Liu JP . (2002). Oncogene 21: 3130–3138.
Castedo M, Ferri K, Roumier T, Metivier D, Zamzami N, Kroemer G . (2002a). J Immunol Methods 265: 39–47.
Castedo M, Hirsch T, Susin SA, Zamzami N, Marchetti P, Macho A et al. (1996). J Immunol 157: 512–521.
Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Andreau K et al. (2002b). EMBO J 21: 4070–4080.
Danial NN, Korsmeyer S . (2004). Cell 116: 205–219.
Debatin KM, Poncet D, Kroemer G . (2002). Oncogene 21: 8786–8803.
Del Bufalo D, Rizzo A, Trisciuoglio D, Cardinali G, Torrisi MR, Zangemeister-Wittke U et al. (2005). Cell Death Differ 12: 1429–1438.
DePinho RA . (2000). Nature 408: 248–254.
Folini M, Brambilla C, Villa R, Gandellini P, Vignati S, Paduano F et al. (2005). Eur J Cancer 41: 624–634.
Goldmacher VS . (2005). Apoptosis 10: 251–265.
Goldmacher VS, Bartle LM, Skletskaya S, Dionne CA, Kedersha NL, Vater CA et al. (1999). Proc Natl Acad Sci USA 96: 12536–12541.
Gorbunova V, Seluanov A, Pereira-Smith OM . (2003). J Biol Chem 278: 7692–7698.
Green DR, Kroemer G . (2004). Science 305: 626–629.
Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM et al. (2004). Circ Res 94: 768–775.
Haendeler J, Hoffmann J, Rahman S, Zeiher AM, Dimmeler S . (2003). FEBS Lett 536: 180–186.
Hanahan D, Weinberg RA . (2000). Cell 100: 57–70.
Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K . (2001). J Cell Sci 114: 4557–4565.
Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M . (2000). Proc Natl Acad Sci USA 97: 10872–10877.
Ito H, Kanzawa T, Miyoshi T, Hirohata S, Kyo S, Iwamaru A et al. (2005). Hum Gene Ther 16: 685–698.
Jacob D, Davis JJ, Zhang L, Zhu H, Teraishi F, Fang B . (2005). Cancer Gene Ther 12: 109–115.
Jiang F, Bao J, Li P, Nicosia SV, Bai W . (2004). J Biol Chem 279: 53213–53221.
Kang HJ, Choi YS, Hong SB, Kim KW, Woo RS, Won SJ et al. (2004). J Neurosci 24: 1280–1287.
Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F et al. (2004). Clin Cancer Res 10: 285–292.
Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV et al. (1999). Science 285: 1733–1737.
Komata T, Kanzawa T, Kondo Y, Kondo S . (2002). Oncogene 21: 656–663.
Lantuejoul S, Soria JC, Morat L, Lorimier P, Moro-Sibilot D, Sabatier L et al. (2005). Clin Cancer Res 11: 2074–2082.
Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H . (2002). Nat Genet 32: 107–108.
Li S, Crothers J, Haqq CM, Blackburn EH . (2005). J Biol Chem 280: 23709–23717.
Li S, Rosenberg JE, Donjacour AA, Botchkina IL, Hom YK, Cunha GR et al. (2004). Cancer Res 64: 4833–4840.
Lin T, Huang X, Gu J, Zhang L, Roth JA, Xiong M et al. (2002). Oncogene 21: 8020–8028.
Lu C, Fu W, Mattson MP . (2001). Brain Res Dev Brain Res 131: 167–171.
Luiten RM, Pene J, Yssel H, Spits H . (2003). Blood 101: 4512–4519.
Masutomi K, Possemato R, Wong JM, Currier JL, Tothova Z, Manola JB et al. (2005). Proc Natl Acad Sci USA 102: 8222–8227.
Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB et al. (2003). Cell 114: 241–253.
Nicoletti I, Migliorati G, Pagliacci MC, Riccardi C . (1991). J Immunol Methods 139: 271–280.
Perfettini JL, Kroemer RT, Kroemer G . (2004). Nat Cell Biol 6: 386–388.
Peter ME, Krammer PH . (2003). Cell Death Differ 10: 26–35.
Poncet D, Larochette N, Pauleau AL, Boya P, Jalil AA, Cartron PF et al. (2004). J Biol Chem 279: 22605–22614.
Rahman R, Latonen L, Wiman KG . (2005). Oncogene 24: 1320–1327.
Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B . (2004). Aging Cell 3: 399–411.
Saretzki G, Fischer H, Kaufmann IG, Schewe C, Nadjari B, Blohmer J et al. (2001). Anal Cell Pathol 23: 39–43.
Schulze-Bergkamen H, Krammer PH . (2004). Semin Oncol 31: 90–119.
Smith LL, Coller HA, Roberts JM . (2003). Nat Cell Biol 5: 474–479.
Stewart SA, Hahn WC, O'Connor BF, Banner EN, Lundberg AS, Modha P et al. (2002). Proc Natl Acad Sci USA 99: 12606–12611.
Takakura M, Kyo S, Inoue M, Wright WE, Shay JW . (2005). Mol Cell Biol 25: 8037–8043.
Uziel O, Fenig E, Nordenberg J, Beery E, Reshef H, Sandbank J et al. (2005). Br J Cancer 92: 1881–1891.
Vogelstein B, Lane D, Levine AJ . (2000). Nature 408: 307–310.
Vousden KH, Lu X . (2002). Nat Rev Cancer 2: 594–604.
Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi X-G, Youle RJ . (1997). J Cell Biol 139: 1281–1292.
Wu P, Meng L, Wang H, Zhou J, Xu G, Wang S et al. (2005). Biochem Biophys Res Commun 335: 36–44.
Xu D, Wang Q, Gruber A, Bjorkholm M, Chen Z, Zaid A et al. (2000). Oncogene 19: 5123–5133.
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssière J-L, Petit PX et al. (1995). J Exp Med 181: 1661–1672.
Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B . (2000). Science 290: 989–992.
Zhang P, Chan SL, Fu W, Mendoza M, Mattson MP . (2003). FASEB J 17: 767–769.
Zhou C, Gehrig PA, Whang YE, Boggess JF . (2003). Mol Cancer Ther 2: 789–795.
Acknowledgements
We thank Dr B Vogelstein (Baltimore, MD) for HCT116 cell lines. This work was supported by grants from French League against Cancer and European Union (RIGHT, ACTIVE p53) to GK and a BQR grant from Paris University XI to J-CS and GK.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Massard, C., Zermati, Y., Pauleau, AL. et al. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene 25, 4505–4514 (2006). https://doi.org/10.1038/sj.onc.1209487
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209487
Keywords
This article is cited by
-
Human telomerase reverse transcriptase positively regulates mitophagy by inhibiting the processing and cytoplasmic release of mitochondrial PINK1
Cell Death & Disease (2020)
-
Exposure to environmental radionuclides associates with tissue-specific impacts on telomerase expression and telomere length
Scientific Reports (2019)
-
Association Between the Telomerase rs2736098_TT Genotype and a Lower Risk of Chronic Hepatitis B and Cirrhosis in Chinese Males
Clinical and Translational Gastroenterology (2017)
-
Telomerase and drug resistance in cancer
Cellular and Molecular Life Sciences (2017)
-
hTERT promotes cell adhesion and migration independent of telomerase activity
Scientific Reports (2016)