Abstract
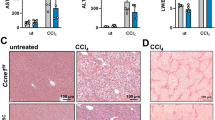
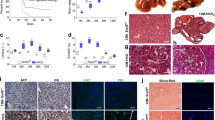
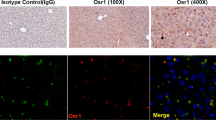
Recently, DNA methylation and reduced expression of the suppressor of the cytokine signaling-3 (SOCS3) gene in human hepatocellular carcinoma (HCC) patients have been reported. However, the roles of SOCS3 in HCC development in vivo have not been clarified. Using RT–PCR analysis and Western blotting, we confirmed that SOCS3 expression was reduced in HCC patients. However, reduced expression of SOCS3 occurred not only in HCC but also in nontumor regions, and this reduction was stronger as the fibrosis grade increased. Furthermore, SOCS3 levels were inversely correlated with signal transducers and activators of transcription-3 (STAT3) activation as well as transforming growth factor (TGF)-β1 levels in the non-HCC region. To define the molecular consequences of SOCS3 silencing/STAT3 hyperactivation and liver fibrosis, we examined liver-specific SOCS3-deficient mice. We demonstrated that SOCS3 deletion in the liver resulted in hyperactivation of STAT3 and promoted ConA- and chemical-induced liver fibrosis. The expression of TGF-β1, a mediator of fibrosis, was enhanced by SOCS3 gene deletion, but suppressed by the overexpression of a dominant-negative STAT3 or SOCS3 both in vivo and in vitro. These data suggest that TGF-β1 is a target gene of STAT3 and could be one of the mechanisms for enhanced fibrosis in SOCS3-deficient mice. Thus, our present study provides a novel role of SOCS3 and STAT3 in HCC development: in addition to the previously characterized oncogenic potentials, STAT3 enhances hepatic fibrosis through the upregulation of TGF-β1 expression, and SOCS3 prevents this process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- JAK:
-
Janus kinase
- STAT:
-
signal transducers and activators of transcription
- SOCS:
-
suppressor of cytokine signaling
- TGF:
-
transforming growth factor
- AST:
-
aspartate aminotransferase
- ALT:
-
alanine aminotransferase
References
Bedossa P, Poynard T . (1996). Hepatology 24: 289–293.
Bissell DM . (2001). Exp Mol Med 33: 179–190.
Bissell DM, Wang SS, Jarnagin WR, Roll FJ . (1995). J Clin Invest 96: 447–455.
Bode JG, Nimmesgern A, Schmitz J, Schaper F, Schmitt M, Frisch W et al. (1999). FEBS Lett 463: 365–370.
Boer AK, Drayer AL, Rui H, Vellenga E . (2002). Blood 100: 467–473.
Bowman T, Garcia R, Turkson J, Jove R . (2000). Oncogene 19: 2474–2488.
Bromberg J, Darnell Jr JE . (2000). Oncogene 19: 2468–2473.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. (1999). Cell 98: 295–303.
Choi I, Kang HS, Yang Y, Pyun KH . (1994). Clin Exp Immunol 95: 530–535.
Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG et al. (2003). Nat Immunol 4: 540–545.
Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L et al. (2004). Immunity 20: 153–165.
Factor VM, Kao CY, Santoni-Rugiu E, Woitach JT, Jensen MR, Thorgeirsson SS . (1997). Cancer Res 57: 2089–2095.
Gorelik L, Flavell RA . (2001). Nat Med 7: 1118–1122.
He B, You L, Uematsu K, Zang K, Xu Z, Lee AY et al. (2003). Proc Natl Acad Sci USA 100: 14133–14138.
Huang KC, Chen CW, Chen JC, Lin WW . (2003). FEBS Lett 555: 385–389.
Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P et al. (2005). Nat Med 11: 845–852.
Jo D, Liu D, Yao S, Collins RD, Hawiger J . (2005). Nat Med 11: 892–898.
Kamio M, Yoshida T, Ogata H, Douchi T, Nagata Y, Inoue M et al. (2004). Oncogene 23: 3107–3115.
Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M et al. (2001). J Biol Chem 276: 12530–12538.
Kamura T, Sato S, Haque D, Liu L, Kaelin Jr WG, Conaway RC et al. (1998). Genes Dev 12: 3872–3881.
Kimura A, Kinjyo I, Matsumura Y, Mori H, Mashima R, Harada M et al. (2004). J Biol Chem 279: 6905–6910.
Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M et al. (2002). Immunity 17: 583–591.
Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J et al. (1995). Science 268: 1336–1338.
Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H et al. (2004). Nat Med 10: 739–743.
Nakamura T, Sakata R, Ueno T, Sata M, Ueno H . (2000). Hepatology 32: 247–255.
Natsume M, Tsuji H, Harada A, Akiyama M, Yano T, Ishikura H et al. (1999). J Leukoc Biol 66: 601–608.
Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T et al. (2005). Oncogene 24: 6406–6417.
Postic C, Magnuson MA . (2000). Genesis 26: 149–150.
Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA et al. (2001). Proc Natl Acad Sci USA 98: 9324–9329.
Saeki A, Tamura S, Ito N, Kiso S, Matsuda Y, Yabuuchi I et al. (2000). Cancer 88: 1025–1029.
Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA . (2002). Hepatology 35: 762–771.
Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K et al. (2001). J Clin Invest 108: 1781–1788.
Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ et al. (2002). Nat Med 8: 1089–1097.
Theiss AL, Fuller CR, Simmons JG, Liu B, Sartor RB, Lund PK . (2005). Gastroenterology 129: 204–219.
Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S et al. (2004). Nat Med 10: 48–54.
Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A et al. (2005). Oncogene 24: 6699–6708.
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC et al. (2003). Oncogene 22: 319–329.
Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D et al. (2003). Nat Immunol 4: 551–556.
Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M et al. (2002). J Exp Med 196: 641–653.
Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y et al. (2004). J Exp Med 199: 1701–1707.
Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE et al. (2001). Nat Genet 28: 29–35.
Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T . (2005). Arthritis Res Ther 7: 100–110.
Acknowledgements
We thank Y Kawabata and T Yoshioka for their help, Mr T Kinoshita, Ms M Othsu, Ms Y Yamada, Mr M Sasaki, and Ms E Fujimoto (Technical Support Center, Medical Institute of Bioregulation) for technical assistance, and Y Nishi for manuscript preparation. This work was supported by special grants-in-aid from the Ministry of Education, Science, Technology, Sports, and Culture of Japan, the Haraguchi Memorial Foundation, the Yamanouchi Foundation for Research on Metabolic Disorders, the Takeda Science Foundation, the Mochida Memorial Foundation, the Kato Memorial Foundation, and the Uehara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogata, H., Chinen, T., Yoshida, T. et al. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-β1 production. Oncogene 25, 2520–2530 (2006). https://doi.org/10.1038/sj.onc.1209281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209281
Keywords
This article is cited by
-
STAT proteins in cancer: orchestration of metabolism
Nature Reviews Cancer (2023)
-
Nintedanib induces senolytic effect via STAT3 inhibition
Cell Death & Disease (2022)
-
Verapamil inhibited the development of ureteral stricture by blocking CaMK II-mediated STAT3 and Smad3/JunD pathways
International Urology and Nephrology (2022)
-
STAT3/HIF-1α signaling activation mediates peritoneal fibrosis induced by high glucose
Journal of Translational Medicine (2021)
-
IL-35 subunit EBI3 alleviates bleomycin-induced pulmonary fibrosis via suppressing DNA enrichment of STAT3
Respiratory Research (2021)