Abstract
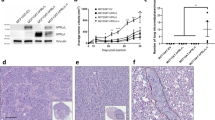
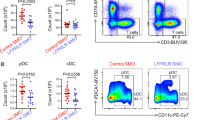
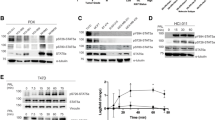
The role of the hormone prolactin (PRL) in the pathogenesis of breast cancer is mediated by its cognate receptor (PRLr). Ubiquitin-dependent degradation of the PRLr that negatively regulates PRL signaling is triggered by PRL-mediated phosphorylation of PRLr on Ser349 followed by the recruitment of the beta-transducin repeats-containing protein (β-TrCP) ubiquitin-protein isopeptide ligase. We report here for the first time that interaction between PRLr and β-TrCP is less efficient in human breast cancer cells than in non-tumorigenic human mammary epithelial cells. Furthermore, we demonstrate that both PRLr degradation and PRLr phosphorylation on Ser349 are impaired in breast tumor cells and tissues, an observation that directly correlates with enhanced expression of the PRLr in malignant breast epithelium. These findings represent a novel mechanism through which altered PRLr stability may directly influence the pathogenesis of breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- PRL:
-
prolactin
- PRLr:
-
prolactin receptor
- β-TrCP:
-
beta-transducin repeats-containing protein
- E3:
-
ubiquitin-protein isopeptide ligase
- HMEC:
-
human mammary epithelial cells
- Stat:
-
signal transducer and activator of transcription
- ICD:
-
intracellular domain
References
Canbay E, Degerli N, Gulluoglu BM, Kaya H, Sen M, Bardakci F . (2004). Curr Med Res Opin 20: 533–540.
Cartun RW, Pedersen CA . (1989). J Histotechnol 12: 273–280.
Clevenger CV . (2004). Am J Pathol 165: 1449–1460.
Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE . (1995). Am J Pathol 146: 695–705.
Clevenger CV, Furth PA, Hankinson SE, Schuler LA . (2003). Endocr Rev 24: 1–27.
Das R, Vonderhaar BK . (1997). J Mammary Gland Biol Neoplasia 2: 29–39.
Diehl JA . (2002). Cancer Biol Ther 1: 226–231.
Glasow A, Horn LC, Taymans SE, Stratakis CA, Kelly PA, Kohler U et al. (2001). J Clin Endocrinol Metab 86: 3826–3832.
Goffin V, Bernichtein S, Touraine P, Kelly PA . (2005). Endocr Rev 26: 400–422.
Harvey PW . (2005). J Appl Toxicol 25: 179–183.
Kim H, Farris J, Christman SA, Kong BW, Foster LK, O’Grady SM et al. (2002). Biochem J 365: 765–772.
Kline JB, Rycyzyn MA, Clevenger CV . (2002). Mol Endocrinol 16: 2310–2322.
Li Y, Kumar KG, Tang W, Spiegelman VS, Fuchs SY . (2004). Mol Cell Biol 24: 4038–4048.
Meng J, Tsai-Morris CH, Dufau ML . (2004). Cancer Res 64: 5677–5682.
Perks CM, Keith AJ, Goodhew KL, Savage PB, Winters ZE, Holly JM . (2004). Br J Cancer 91: 305–311.
Polakis P . (1999). Curr Opin Genet Dev 9: 15–21.
Polakis P . (2000). Genes Dev 14: 1837–1851.
Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV . (1997). Endocrinology 138: 5555–5560.
Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA . (2003). Oncogene 22: 4664–4674.
Spiegelman VS, Tang W, Chan AM, Igarashi M, Aaronson SA, Sassoon DA et al. (2002). J Biol Chem 277: 36624–36630.
Tang W, Li Y, Yu D, Thomas-Tikhonenko A, Spiegelman VS, Fuchs SY . (2005). Cancer Res 65: 1904–1908.
Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE . (2004). Cancer Res 64: 6814–6819.
Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Tornell J . (1997). J Clin Invest 100: 2744–2751.
Wennbo H, Tornell J . (2000). Oncogene 19: 1072–1076.
Acknowledgements
We thank Drs Ronai, Foster, and Parlow for providing reagents and CHTN and the Tissue Resource Core of the Breast SPORE of the Robert H Lurie Comprehensive Cancer Center of Northwestern University (Chicago, IL, USA) for primary tissues and the array. This work was supported in part by The University of Pennsylvania Cancer Center Pilot Grant and The Susan G Komen Breast Cancer Foundation grant BCTR0504447 (to SYF) and NCI Grant CA069294 (to CVC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Clevenger, C., Minkovsky, N. et al. Stabilization of prolactin receptor in breast cancer cells. Oncogene 25, 1896–1902 (2006). https://doi.org/10.1038/sj.onc.1209214
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209214
Keywords
This article is cited by
-
The human intermediate prolactin receptor is a mammary proto-oncogene
npj Breast Cancer (2021)
-
A growth hormone receptor SNP promotes lung cancer by impairment of SOCS2-mediated degradation
Oncogene (2018)
-
Endocrine control of canine mammary neoplasms: serum reproductive hormone levels and tissue expression of steroid hormone, prolactin and growth hormone receptors
BMC Veterinary Research (2015)
-
Prolactin and Prolactin Receptor Expression in Cervical Intraepithelial Neoplasia and Cancer
Pathology & Oncology Research (2015)
-
Inflammatory signaling compromises cell responses to interferon alpha
Oncogene (2012)