Abstract
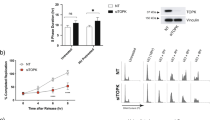
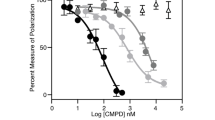
Checkpoint kinase 2 (Chk2) is known to mediate diverse cellular responses to genotoxic stress. The fundamental role of Chk2 is to regulate the network of genome-surveillance pathways that coordinate cell-cycle progression with DNA repair and cell survival or death. Defects in Chk2 contribute to the development of both hereditary and sporadic human cancers. We now present evidence that the human T-cell leukemia virus type-1 (HTLV-1) Tax protein directly interacts with Chk2 and the kinase activity of Chk2 is inhibited by Tax. The physical interaction of Chk2 and Tax was observed by co-immunoprecipitation assays in HTLV-1-infected T cells (C81) as well as GST pull-down assays using purified proteins. Binding and kinase activity inhibition studies with Tax deletion mutants indicated that at least two domains of Tax mediate the interaction with Chk2. We have analysed the functional consequence of de novo expression of Tax upon the cellular DNA-damage-induced apoptosis, which is mediated by Chk2. Using transient transfection and TUNEL assay, we found that γ-irradiation-induced apoptosis was decreased in 293T and HCT-116 (p53−/−) cells expressing HTLV-1 Tax. Our studies demonstrate an important potential target of Tax in cellular transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ahn J, Urist M, Prives C . (2004). DNA Repair 3: 1039–1047.
Akagi T, Ono H, Tsuchida N, Shimotohno K . (1997). FEBS Lett 406: 263–266.
Anderson CW, Appella E, Sakaguchi K . (1998). J Protein Chem 17: 527.
Ariumi Y, Ego T, Kaida A, Matsumoto M, Pandolfi PP, Shimotohno K . (2003). Oncogene 22: 1611–1619.
Ariumi Y, Kaida A, Lin JY, Hirota M, Masui O, Yamaoka S et al. (2000). Oncogene 19: 1491–1499.
Bargonetti J, Reynisdottir I, Friedman PN, Prives C . (1992). Genes Dev 6: 1886–1898.
Bartek J, Lukas J . (2003). Cancer Cell 3: 421–429.
Bex F, Gaynor RB . (1998). Methods 16: 83–94.
Brown AL, Lee CH, Schwarz JK, Mitiku N, Piwnica-Worms H, Chung JH . (1999). Proc Natl Acad Sci USA 96: 3745–3750.
Burton M, Upadhyaya CD, Maier B, Hope TJ, Semmes OJ . (2000). J Virol 74: 2351–2364.
Cereseto A, Diella F, Mulloy JC, Cara A, Michieli P, Grassmann R et al. (1996). Blood 88: 1551–1560.
Coogan TP, Bare RM, Bjornson EJ, Waalkes MP . (1994). J Toxicol Environ Health 41: 233–245.
Coogan TP, Bare RM, Waalkes MP . (1992). Toxicol Appl Pharmacol 113: 227–233.
Coscoy L, Gonzalez-Dunia D, Tangy F, Syan S, Brahic M, Ozden S . (1998). Virology 248: 332–341.
Franchini G . (1995). Blood 86: 3619–3639.
Gartenhaus RB, Wang P . (1995). Leukemia 9: 2082–2086.
Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG et al. (1992). J Virol 66: 4570–4575.
Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar MC et al. (1989). Proc Natl Acad Sci USA 86: 3351–3355.
Grossman WJ, Kimata JT, Wong F, Zutter M, Ley TJ, Ratner L . (1995). Proc Natl Acad Sci USA 92: 1057–1061.
Haoudi A, Semmes OJ . (2003). Virology 305: 229–239.
Haoudi A, Daniels RC, Wong E, Kupfer G, Semmes OJ . (2003). J Biol Chem 278: 37736–37744.
Higuchi R . (1990). Recombinant PCR: A Guide to Methods and Applications. San Diego, CA: Academic Press, pp. 177–183.
Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A et al. (2002). Mol Cell Biol 22: 6521–6532.
Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H et al. (2000). Science 287: 1824–1827.
Hollsberg P . (1999). Microbiol Mol Biol Rev 63: 308–333.
Jack MT, Woo RA, Hirao A, Cheung A, Mak TW, Lee PWK . (2002). Proc Natl Acad Sci USA 99: 9825–9829.
Jallepalli PV, Lengauer C, Vogelstein B, Bunz F . (2003). J Biol Chem 278: 20475–20479.
Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN . (2005). J Biol Chem 280: 10326–10332.
Jeong SJ, Radonovich M, Brady JN, Pise-Masison CA . (2004). Blood 104: 1490–1497.
Johnson JM, Harrod R, Franchini G . (2001). Int J Exp Pathol 82: 135–147.
Lemoine FJ, Marriott SJ . (2001). J Biol Chem 276: 31851–31857.
Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AEH, Yaffe MB . (2005). Mol Cell 17: 37–48.
Matsuda T, Almasan A, Tomita M, Uchihara Jn, Masuda M, Ohshiro K et al. (2005). J Virol 79: 1367–1378.
McGowan CH . (2002). Bioessays 24: 502–511.
Mulloy JC, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J et al. (1998). J Virol 72: 8852–8860.
O'Neill T, Giarratani L, Chen P, Iyer L, Lee CH, Bobiak M et al. (2002). J Biol Chem 277: 16102–16115.
Park HU, Jeong JH, Chung JH, Brady JN . (2004). Oncogene 23: 4966–4974.
Peters M, DeLuca C, Hirao A, Stambolic V, Potter J, Zhou L et al. (2002). Proc Natl Acad Sci USA 99: 11305–11310.
Pise-Masison CA, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Duvall J et al. (2000). Mol Cell Biol 20: 3377–3386.
Pise-Masison CA, Mahieux R, Radonovich M, Jiang H, Brady JN . (2001). J Biol Chem 276: 200–205.
Pise-Masison CA, Radonovich M, Sakaguchi K, Appella E, Brady JN . (1998). J Virol 72: 6348–6355.
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC . (1980). Proc Natl Acad Sci USA 77: 7415–7419.
Portis T, Grossman WJ, Harding JC, Hess JL, Ratner L . (2001a). J Virol 75: 2185–2193.
Portis T, Harding JC, Ratner L . (2001b). Blood 98: 1200–1208.
Robek MD, Ratner L . (1999). J Virol 73: 4856–4865.
Segawa K, Minowa A, Sugasawa K, Takano T, Hanaoka F . (1993). Oncogene 8: 543–548.
Semmes OJ, Jeang KT . (1996). J Virol 70: 6347–6357.
Seo GJ, Kim SE, Lee YM, Lee JW, Lee JR, Hahn MJ et al. (2003). Bio Biophys Res Commun 304: 339–343.
Sheppard HM, Corneillie SI, Espiritu C, Gatti A, Liu X . (1999). Mol Cell Biol 19: 2746–2753.
Sieburg M, Tripp A, Ma JW, Feuer G . (2004). J Virol 78: 10399–10409.
Smith MR, Greene WC . (1990). Genes Dev 4: 1875–1885.
Stevens C, Smith L, La Thangue NB . (2003). Nat Cell Biol 5: 401–409.
Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S et al. (2002). EMBO J 21: 5195–5205.
Tanaka A, Takahashi G, Yamaoka S, Nosaka T, Maki M, Hatanaka M . (1990). Proc Natl Acad Sci USA 87: 1071–1075.
Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G et al. (1999). J Virol 73: 7981–7987.
Urist M, Tanaka T, Poyurovsky MV, Prives C . (2004). Genes Dev 18: 3041–3054.
Van Orden K, Giebler HA, Lemasson I, Gonzales M, Nyborg JK . (1999). J Biol Chem 274: 26321–26328.
Yang S, Kuo C, Bisi JE, Kim MK . (2002). Nat Cell Biol 4: 865–870.
Zhou BB, Bartek J . (2004). Nat Rev Cancer 4: 216–225.
Acknowledgements
This research was supported by the intramural Research Program of the NIH, National Cancer Institute. We thank the FACS and Image core facility of CCR, NCI, NIH for flow cytometry analysis and confocal microscopy, respectively. We also thank Drs Bert Vogelstein and Fred Bunz for providing HCT-116 p21+/+ and p53−/− cell lines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, H., Jeong, SJ., Jeong, JH. et al. Human T-cell leukemia virus type 1 Tax attenuates γ-irradiation-induced apoptosis through physical interaction with Chk2. Oncogene 25, 438–447 (2006). https://doi.org/10.1038/sj.onc.1209059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209059
Keywords
This article is cited by
-
How the DNA damage response determines the fate of HTLV-1 Tax-expressing cells
Retrovirology (2012)
-
The DNA damage response in viral-induced cellular transformation
British Journal of Cancer (2012)
-
Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ and therapy
Oncogene (2011)
-
The HTLV-1 Tax interactome
Retrovirology (2008)
-
Telomere attrition induces a DNA double-strand break damage signal that reactivates p53 transcription in HTLV-I leukemic cells
Oncogene (2008)