Abstract
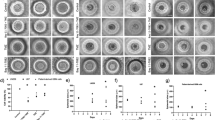
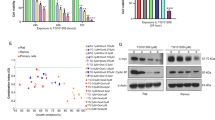
Recent evidence demonstrates that the anticancer activity of betulinic acid (BetA) can be markedly increased by combination protocols, for example with chemotherapy, ionizing radiation or TRAIL. Since nuclear factor-kappaB (NF-κB), a key regulator of stress-induced transcriptional activation, has been implicated in mediating apoptosis resistance, we investigated the role of NF-κB in BetA-induced apoptosis. Here, we provide for the first time evidence that BetA activates NF-κB in a variety of tumor cell lines. NF-κB DNA-binding complexes induced by BetA consisted of p50 and p65 subunits. Nuclear translocation of p65 was also confirmed by immunofluorescence microscopy. BetA-induced NF-κB activation involved increased IKK activity and phosphorylation of IκB-α at serine 32/36 followed by degradation of IκB-α. Reporter assays revealed that NF-κB activated by BetA is transcriptionally active. Interestingly, inhibition of BetA-induced NF-κB activation by different chemical inhibitors (proteasome inhibitor, antioxidant, IKK inhibitor) attenuated BetA-induced apoptosis. Importantly, specific NF-κB inhibition by transient or stable expression of IκB-α super-repressor inhibited BetA-induced apoptosis in SH-EP neuroblastoma cells, while transient expression of IκB-α super-repressor had no influence on BetA-induced apoptosis in two other cell lines. Thus, our findings that activation of NF-κB by BetA promotes BetA-induced apoptosis in a cell type-specific fashion indicate that NF-κB inhibitors in combination with BetA would have no therapeutic benefit or could even be contraproductive in certain tumors, which has important implications for the design of BetA-based combination protocols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- AIF:
-
apoptosis-inducing factor
- BetA:
-
betulinic acid
- FACS:
-
fluorescence-activated cell-sorting
- GST:
-
gluthathione-S-transferase
- IAPs:
-
inhibitor of apoptosis proteins
- IκB:
-
inhibitor of κB
- IKK:
-
IκB kinase
- NF-κB:
-
nuclear factor-kappaB
- PDTC:
-
pyrrolidine dithiocarbamate
- Smac:
-
second mitochondria-derived activator of caspase
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- XIAP:
-
X-linked inhibitor of apoptosis
- zVAD.fmk:
-
benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
References
Algul H, Adler G and Schmid RM . (2002). Int. J. Gastrointest. Cancer, 31, 71–78.
Baetu TM, Kwon H, Sharma S, Grandvaux N and Hiscott J . (2001). J. Immunol., 167, 3164–3173.
Baumann B, Bohnenstengel F, Siegmund D, Wajant H, Weber C, Herr I, Debatin KM, Proksch P and Wirth T . (2002). J. Biol. Chem., 277, 44791–44800.
Campbell KJ, Rocha S and Perkins ND . (2004). Mol. Cell, 13, 853–865.
Debatin KM, Poncet D and Kroemer G . (2002). Oncogene, 21, 8786–8803.
Fulda S and Debatin KM . (2002). Oncogene, 21, 2295–2308.
Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, Nunez G, Krammer PH, Peter ME and Debatin KM . (1997). Cancer Res., 57, 4956–4964.
Fulda S, Jeremias I and Debatin KM . (2004). Oncogene, 23, 7611–7620.
Fulda S, Jeremias I, Steiner HH, Pietsch T and Debatin KM . (1999). Int. J. Cancer, 82, 435–441.
Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME and Debatin KM . (1998a). J. Biol. Chem., 273, 33942–33948.
Fulda S, Susin SA, Kroemer G and Debatin KM . (1998b). Cancer Res., 58, 4453–4460.
Hayden MS and Ghosh S . (2004). Genes Dev., 18, 2195–2224.
Hengartner MO . (2000). Nature, 407, 770–776.
Herr I and Debatin KM . (2001). Blood, 98, 2603–2614.
Jeremias I, Kupatt C, Baumann B, Herr I, Wirth T and Debatin KM . (1998). Blood, 91, 4624–4631.
Johnstone RW, Ruefli AA and Lowe SW . (2002). Cell, 108, 153–164.
Karin M, Cao Y, Greten FR and Li ZW . (2002). Nat. Rev. Cancer, 2, 301–310.
Karin M, Yamamoto Y and Wang QM . (2004). Nat. Rev. Drug Disc., 3, 17–26.
Kim B and Feldman EL . (2002). J. Biol. Chem., 277, 27393–27400.
Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, Wani MC, Wall ME, Hieken TJ, Das Gupta TK and Pezzuto JM . (1995). Nat. Med., 1, 1046–1051.
Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ and Bedi A . (2001). Nat. Cell Biol., 3, 409–416.
Sawada N, Kataoka K, Kondo K, Arimochi H, Fujino H, Takahashi Y, Miyoshi T, Kuwahara T, Monden Y and Ohnishi Y . (2004). Br. J. Cancer., 90, 1672–1678.
Selzer E, Pimentel E, Wacheck V, Schlegel W, Pehamberger H, Jansen B and Kodym R . (2000). J. Invest. Dermatol., 114, 935–940.
Takada Y and Aggarwal BB . (2003). J. Immunol., 171, 3278–3286.
van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ and Vandenabeele P . (2002). Cell Death Differ., 9, 1031–1042.
Wahl C, Liptay S, Adler G and Schmid RM . (1998). J. Clin. Invest., 101, 1163–1174.
Weaver KD, Yeyeodu S, Cusack Jr JC, Baldwin Jr AS and Ewend MG . (2003). J. Neuro-Oncol., 61, 187–196.
Younes A, Garg A and Aggarwal BB . (2003). Leuk. Lymphoma, 44, 929–935.
Zhang Y and Chen F . (2004). Cancer Res., 64, 1902–1905.
Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C and Formelli F . (2002). Cancer Lett., 175, 17–25.
Zwacka RM, Stark L and Dunlop MG . (2000). J. Gene Med., 2, 334–343.
Acknowledgements
We thank S Piater for expert technical assistance and B Baumann (Department of Physiological Chemistry, University of Ulm, Germany) for helpful discussions. This work has been partially supported by grants from the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe, the Bundesministerium für Forschung und Technologie, the Ministry of Science, Research and Arts of Baden-Württemberg, IZKF Ulm, Wilhelm-Sander-Stiftung and Else-Kröner-Stiftung (KMD and SF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasperczyk, H., La Ferla-Brühl, K., Westhoff, M. et al. Betulinic acid as new activator of NF-κB: molecular mechanisms and implications for cancer therapy. Oncogene 24, 6945–6956 (2005). https://doi.org/10.1038/sj.onc.1208842
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208842
Keywords
This article is cited by
-
Glaucocalyxin A delays the progression of OA by inhibiting NF-κB and MAPK signaling pathways
Journal of Orthopaedic Surgery and Research (2024)
-
Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential
Phytochemistry Reviews (2019)
-
Cytokines/Chemokines Profile in Rats Treated with Euphorbia tirucalli Extract
Arabian Journal for Science and Engineering (2018)
-
Overaccumulation of p53-mediated autophagy protects against betulinic acid-induced apoptotic cell death in colorectal cancer cells
Cell Death & Disease (2017)
-
Betulinic acid enhances TGF-β signaling by altering TGF-β receptors partitioning between lipid-raft/caveolae and non-caveolae membrane microdomains in mink lung epithelial cells
Journal of Biomedical Science (2016)