Abstract
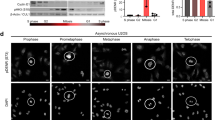
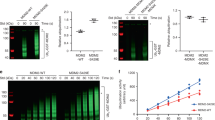
Mdm2 and MdmX function as cellular regulators of the p53 tumor suppressor protein. Intriguingly, the activities of these proteins are interdependent; MdmX stabilizes Mdm2, enabling its activities towards p53, but it also requires Mdm2 for its nuclear localization. Here we demonstrate that via its phosphorylation by CDK2/Cdc2p34, MdmX regulates nuclear export of Mdm2. Cdc2p34 phosphorylates MdmX on Ser 96 in vitro. Mutation within this site (MdmXS96A) impairs, whereas phosphomimic substitution (MdmXS96D) increases the cytoplasmic localization of MdmX, suggesting that CDK2/Cdc2p34 phosphorylation is required for export of MdmX from the nucleus. Consequently, cells that express MdmXS96A retain Mdm2 in their nuclei, suggesting that export of Mdm2 to the cytoplasm is MdmX-dependent. Similarly, treatment of cells with the pharmacological inhibitor of CDK2/Cdc2p34 or with a dominant-negative Cdc2 results in nuclear localization of MdmX and Mdm2 and decreases the level of Mdm2 expression. Since Cdc2p34 is active in nonstressed conditions, our finding provides a novel insight into the signaling cascade involved in the regulation of MdmX localization and for regulation of Mdm2 localization and stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alarcon-Vargas D, Takahashi S and Ronai Z . (2003). Adv. Cancer Res., 89, 1–34.
Badciong JC and Haas AL . (2002). J. Biol. Chem., 277, 49668–49675.
Danovi D, Meulmeester E, Pasini D, Migliorini D, Capra M, Frenk R, de Graaf P, Francoz S, Gasparini P, Gobbi A, Helin K, Pelicci PG, Jochemsen AG and Marine JC . (2004). Mol. Cell. Biol., 24, 5835–5843.
Fang S, Jensen JP, Ludwig RL, Vousden KH and Weissman AM . (2000). J. Biol. Chem., 275, 8945–8951.
Finch RA, Donoviel DB, Potter D, Shi M, Fan A, Freed DD, Wang CY, Zambrowicz BP, Ramirez-Solis R, Sands AT and Zhang N . (2002). Cancer Res., 62, 3221–3225.
Fuchs SY, Adler V, Buschmann T, Wu X and Ronai Z . (1998). Oncogene, 17, 2543–2547.
Ghosh M, Huang K and Berberich SJ . (2003). Biochemistry, 42, 2291–2299.
Gu J, Kawai H, Nie L, Kitao H, Wiederschain D, Jochemsen AG, Parant J, Lozano G and Yuan ZM . (2002). J. Biol. Chem., 277, 19251–19254.
Haupt Y, Maya R, Kazaz A and Oren M . (1997). Nature, 387, 296–299.
Honda R and Yasuda H . (2000). Oncogene, 19, 1473–1476.
Jackson MW and Berberich SJ . (1999). DNA Cell. Biol., 18, 693–700.
Li C, Chen L and Chen J . (2002). Mol. Cell. Biol., 22, 7562–7571.
Li M, Brooks CL, Wu-Baer F, Chen D, Baer R and Gu W . (2003). Science, 302, 1972–1975.
Migliorini D, Danovi D, Colombo E, Carbone R, Pelicci PG and Marine JC . (2002a). J. Biol. Chem., 277, 7318–7323.
Migliorini D, Denchi EL, Danovi D, Jochemsen A, Capillo M, Gobbi A, Helin K, Pelicci PG and Marine JC . (2002b). Mol. Cell. Biol., 22, 5527–5538.
Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG and Lozano G . (2001). Nat. Genet., 29, 92–95.
Parant JM, Reinke V, Mims B and Lozano G . (2001). Gene, 270, 277–283.
Ramos YF, Stad R, Attema J, Peltenburg LT, van der Eb AJ and Jochemsen AG . (2001). Cancer Res., 61, 1839–1842.
Riemenschneider MJ, Buschges R, Wolter M, Reifenberger J, Bostrom J, Kraus JA, Schlegel U and Reifenberger G . (1999). Cancer Res., 59, 6091–6096.
Sabbatini P and McCormick F . (2002). DNA Cell. Biol., 21, 519–525.
Shah OJ, Ghosh S and Hunter T . (2003). J. Biol. Chem., 278, 16433–16442.
Sharp DA, Kratowicz SA, Sank MJ and George DL . (1999). J. Biol. Chem., 274, 38189–38196.
Shvarts A, Bazuine M, Dekker P, Ramos YF, Steegenga WT, Merckx G, van Ham RC, van der Houven van Oordt W, van der Eb AJ and Jochemsen AG . (1997). Genomics, 43, 34–42.
Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt W, Hateboer G, van der Eb AJ and Jochemsen AG . (1996). EMBO J., 15, 5349–5357.
Stad R, Little NA, Xirodimas DP, Frenk R, van der Eb AJ, Lane DP, Saville MK and Jochemsen AG . (2001). EMBO Rep., 2, 1029–1034.
Tanimura S, Ohtsuka S, Mitsui K, Shirouzu K, Yoshimura A and Ohtsubo M . (1999). FEBS Lett., 447, 5–9.
Zhang T and Prives C . (2001). J. Biol. Chem., 276, 29702–29710.
Acknowledgements
We thank Guillermina Lozano for providing the p53/MdmX and p53/Mdm2 DKO cells, Donna George for MdmX constructs, Ed Harlow for the Cdc2DN construct, Zhimin Yuan for MdmX constructs and AG Jochemsen for MdmX antibodies. We also thank Anindita Bhoumik for help and to Paul Carman for assistance with confocal microscopy. Support by NCI grant (CA59908 to ZR) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elias, B., Laine, A. & Ronai, Z. Phosphorylation of MdmX by CDK2/Cdc2p34 is required for nuclear export of Mdm2. Oncogene 24, 2574–2579 (2005). https://doi.org/10.1038/sj.onc.1208488
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208488
Keywords
This article is cited by
-
Evaluation of novel cell cycle inhibitors in mantle cell lymphoma
Oncogene (2007)
-
14-3-3γ binds to MDMX that is phosphorylated by UV-activated Chk1, resulting in p53 activation
The EMBO Journal (2006)