Abstract
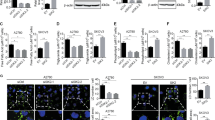
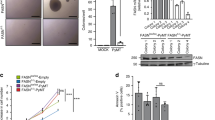
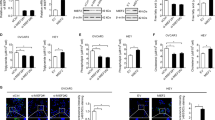
Activation of AKT and overexpression of fatty acid synthase (FAS) are frequently observed in human ovarian cancer. To explore a possible connection between AKT and FAS, immunohistochemical analyses were conducted on an ovarian cancer tissue microarray, which revealed a significant correlation between phosphorylated AKT (phospho-AKT) and expression of FAS. To investigate the relationship between phospho-AKT and FAS in vitro, a variety of experiments employing a specific phosphatidylinositol 3-OH kinase (PI3K) inhibitor (LY294002), inducible PTEN expression in PTEN-null cells, or AKT1 siRNA demonstrated that phosphatidylinositol-3 kinase (PI3K)/AKT signaling modulates FAS expression. In contrast, inhibition of FAS activity by the drug C75 resulted in downregulation of phospho-AKT and increased cell death. To explore the functional relationship between phospho-AKT and FAS, we used SKOV3, C200, and OVCAR10 ovarian carcinoma cells, which have constitutively active AKT, and OVCAR5 cells, which have very low basal phospho-AKT levels. Treatment with LY294002 abolished AKT activity and potentiated apoptosis induced by FAS inhibitors cerulenin or C75 only in cells with constitutively active AKT, suggesting that constitutive activation of AKT protects against FAS inhibitor-induced cell death. Furthermore, inhibition of FAS activity by cerulenin or C75 resulted in downregulation of phospho-AKT, which preceded the induction of apoptosis. To investigate the relationship between phospho-AKT and FAS in vivo, severe combined immunodeficient mice injected intraperitoneally with SKOV3 cells were treated with C75. Growth of SKOV3 xenografts was markedly inhibited by C75. Analysis of the levels of phospho-AKT and FAS in C75-treated tumors revealed concordant downregulation of phospho-AKT and FAS. Collectively, our findings are consistent with a working model in which AKT activation regulates FAS expression, at least in part, whereas FAS activity modulates AKT activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- FAS:
-
fatty acid synthase
- PI3K:
-
phosphatidylinositol 3-OH kinase
- SCID:
-
severe combined immunodeficient
References
Alo PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, Serpieri DE and Di Tondo U . (2000). Oncol. Rep., 7, 1383–1388.
Alo PL, Visca P, Marci A, Mangoni A, Botti C and Di Tondo U . (1996). Cancer, 77, 474–482.
Aunoble B, Sanches R, Didier E and Bignon YJ . (2000). Int. J. Oncol., 16, 567–576.
Brognard J, Clark AS, Ni Y and Dennis PA . (2001). Cancer Res., 61, 3986–3997.
Camassei FD, Cozza R, Acquaviva A, Jenkner A, Rava L, Gareri R, Donfrancesco A, Bosman C, Vadala P, Hadjistilianou T and Boldrini R . (2003a). Invest. Ophthalmol. Vis. Sci., 44, 2399–2403.
Camassei FD, Jenkner A, Rava L, Bosman C, Francalanci P, Donfrancesco A, Alo PL and Boldrini R . (2003b). Med. Pediatr. Oncol., 40, 302–308.
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN and Testa JR . (1992). Proc. Natl. Acad. Sci. USA, 89, 9267–9271.
Clark AS, West K, Streicher S and Dennis PA . (2002). Mol. Cancer Ther., 1, 707–717.
D'Agnolo G, Rosenfeld IS, Awaya J, Omura S and Vagelos PR . (1973). Biochim. Biophys. Acta, 326, 155–156.
Datta SR, Brunet A and Greenberg ME . (1999). Genes Dev., 13, 2905–2927.
De Schrijver E, Brusselmans K, Heyns W, Verhoeven G and Swinnen JV . (2003). Cancer Res., 63, 3799–3804.
Epstein JI, Carmichael M and Partin AW . (1995). Urology, 45, 81–86.
Gabrielson EW, Pinn ML, Testa JR and Kuhajda FP . (2001). Clin. Cancer Res., 7, 153–177.
Gansler TS, Hardman III W, Hunt DA, Schaffel S and Hennigar RA . (1997). Hum. Pathol., 28, 686–692.
Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC and Anderson ME . (1992). Proc. Natl. Acad. Sci. USA, 89, 3070–3074.
Hill MM, Feng J and Hemmings BA . (2002). Curr. Biol., 12, 1251–1255.
Hu L, Hofmann J, Lu Y, Mills GB and Jaffe RB . (2002). Cancer Res., 62, 1087–1092.
Jackowski S, Wang J and Baburina I . (2000). Biochim. Biophys. Acta, 1483, 301–315.
Jin W, Wu L, Liang K, Liu B, Lu Y and Fan Z . (2003). Br. J. Cancer, 89, 185–191.
Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, Schmidt M, Mills GB, Mendelsohn J and Fan Z . (2003). Oncogene, 22, 3205–3212.
Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL and Townsend CA . (2000). Proc. Natl. Acad. Sci. USA, 97, 3450–3454.
Kusakabe T, Nashimoto A, Honma K and Suzuki T . (2002). Histopathology, 40, 71–79.
Ng SSW, Tsao MS, Chow S and Hedley DW . (2000). Cancer Res., 60, 5451–5455.
Page C, Lin HJ, Jin Y, Castle VP, Nunez G, Huang M and Lin J . (2000). Anticancer Res., 20, 407–416.
Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ and Phillips WA . (2001). Cancer Res., 61, 7426–7429.
Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC and Grizzle WE . (2000). Hum. Pathol., 31, 1068–1073.
Pizer ES, Chrest FJ, DiGiuseppe JA and Han WF . (1998). Cancer Res., 58, 4611–4615.
Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE and Kuhajda FP . (1996a). Cancer Res., 56, 2745–2747.
Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS and Nelson JB . (2001). Prostate, 47, 102–110.
Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA and Kuhajda FP . (2000). Cancer Res., 60, 213–218.
Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR and Kuhajda FP . (1996b). Cancer Res., 56, 1189–1193.
Resjo S, Goransson O, Harndahl L, Zolnierowicz S, Manganiello V and Degerman E . (2002). Cell Signal., 14, 231–238.
Sargiacomo M, Sudol M, Tang Z and Lisanti MP . (1993). J. Cell Biol., 122, 789–807.
Schmidt M, Hovelmann S and Beckers TL . (2002). Br. J. Cancer, 87, 924–932.
Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB and Gray JW . (1999). Nat. Genet., 21, 99–102.
Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV and Cheng JQ . (2001). Am. J. Pathol., 159, 431–437.
Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T, Heemers H, Heyns W and Verhoeven G . (2003). Biochem. Biophys. Res. Commun., 302, 898–903.
Testa JR and Bellacosa A . (2001). Proc. Natl. Acad. Sci. USA, 98, 10983–10985.
Thupari JN, Landree LE, Ronnett GV and Kuhajda FP . (2002). Proc. Natl. Acad. Sci. USA, 99, 9498–9502.
Van de Sande T, De Schrijver E, Heyns W, Verhoeven G and Swinnen JV . (2002). Cancer Res., 62, 642–646.
Wang D and Sul HS . (1998). J. Biol. Chem., 273, 25420–25426.
Wang HQ and Smart RC . (1999). J. Cell Sci., 112 (Part 20), 3497–3506.
Yang YA, Han WF, Morin PJ, Chrest FJ and Pizer ES . (2002). Exp. Cell Res., 279, 80–90.
Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV and Cheng JQ . (2000). Oncogene, 19, 2324–2330.
Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV and Kuhajda FP . (2003). Cancer Res., 63, 7330–7337.
Zhuang L, Lin J, Lu ML, Solomon KR and Freeman MR . (2002). Cancer Res., 62, 2227–2231.
Acknowledgements
We thank Dr AJP Klein-Szanto for immunohistochemical analysis and H Wang for statistical analysis. This work was supported by National Cancer Institute Grants CA83638 (SPORE in Ovarian Cancer), CA77429, and CA06927, and by an appropriation from the Commonwealth of Pennsylvania. The following Fox Chase Cancer Center shared facilities were used in the course of this work: Cell Culture Facility, Biosample and Tissue Procurement Core, and Laboratory Animal Facility. HQ Wang is supported by a Board of Directors Postdoctoral Fellowship at Fox Chase Cancer Center and by an NIH Institutional Training Grant CA-09035-28. FASgen did not contribute reagents or support for these studies. However, we acknowledge that under a licensing agreement between FASgen and the Johns Hopkins University, FPK is entitled to a share of royalty received by the University on products that may be developed from FAS technology. FPK owns FASgen stock, which is subject to certain restrictions under University policy. The Johns Hopkins University, in accordance with its conflict of interest policies, is managing the terms of this arrangement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Altomare, D., Skele, K. et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene 24, 3574–3582 (2005). https://doi.org/10.1038/sj.onc.1208463
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208463
Keywords
This article is cited by
-
Cerulenin suppresses ErbB2-overexpressing breast cancer by targeting ErbB2/PKM2 pathway
Medical Oncology (2022)
-
Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt
Cell Death & Disease (2021)
-
Fructose and prostate cancer: toward an integrated view of cancer cell metabolism
Prostate Cancer and Prostatic Diseases (2019)
-
Fatty Acid Inhibition Sensitizes Androgen-Dependent and -Independent Prostate Cancer to Radiotherapy via FASN/NF-κB Pathway
Scientific Reports (2019)
-
Loss of fatty acid synthase suppresses the malignant phenotype of colorectal cancer cells by down-regulating energy metabolism and mTOR signaling pathway
Journal of Cancer Research and Clinical Oncology (2016)