Abstract
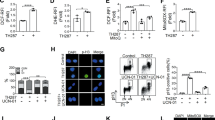
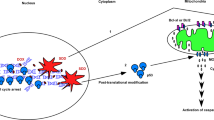
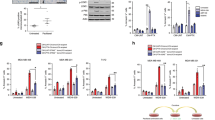
Most cancer therapeutics fails to eradicate cancer because cancer cells rapidly develop resistance to its proapoptotic effects. The underlying mechanisms remain incompletely understood. Here we show that three representative apoptotic stimuli, that is, serum starvation, a mitochondrial toxin, and a DNA-damaging agent (etoposide), rapidly induce several distinct classes of prosurvival molecules, in particular, Bcl-2/Bcl-XL and superoxide dismutase (SOD; including both MnSOD and Cu/ZnSOD). At the population level, the induction of these prosurvival molecules occurs prior to or concomitant with the induction of proapoptotic molecules such as Bim and Bak. Blocking the induction using siRNAs of the prosurvival or proapoptotic molecules facilitates or inhibits apoptosis, respectively. One master transcription factor, FOXO3a, is involved in the transcriptional activation of some of these prosurvival (e.g., MnSOD) and proapoptotic (e.g., Bim) molecules. Interestingly, in all three apoptotic systems, FOXO3a itself is also upregulated at the transcriptional level. Mechanistic studies indicate that reactive oxygen species (ROS) are rapidly induced upon apoptotic stimulation and that ROS inhibitors/scavengers block the induction of FOXO3a, MnSOD, and Bim. Finally, we show that apoptotic stimuli also upregulate prosurvival molecules in normal diploid human fibroblasts and at subapoptotic concentrations. Taken together, these results suggest that various apoptotic inducers may rapidly mobilize prosurvival mechanisms through ROS-activated master transcription factors such as FOXO3a. The results imply that effective anticancer therapeutics may need to combine both apoptosis-inducing and survival-suppressing strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Adler V, Yin Z, Tew KD and Ronai Z . (1999). Oncogene, 18, 6104–6111.
Birkenkamp KU and Coffer PJ . (2003). J. Immunol., 171, 1623–1629.
Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM and Strasser A . (2002). Nature, 415, 922–926.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME . (1999). Cell, 96, 857–868.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng H-L, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FE and Greenberg ME . (2004). Science, 303, 2011–2015.
Castrillon DH, Miao L, Kollipara R, Horner JW and DePinho RA . (2003). Science, 301, 215–218.
Chandra D, Choy G, Deng X, Bhatia B, Daniel P and Tang DG . (2004). Mol. Cell. Biol., 24, 6592–6607.
Chandra D, Liu JW and Tang DG . (2002). J. Biol. Chem., 277, 50842–50854.
Chandra D and Tang DG . (2003). J. Biol. Chem., 278, 17408–17420.
Danial NN and Korsmeyer SJ . (2004). Cell, 116, 205–219.
Dijkers PF, Birkenkamp KU, Lam EW-F, Thomas NSB, Lammers J-W, Koenderman L and Coffer PJ . (2002). J. Cell Biol., 156, 531–542.
Dijkers PF, Medema RH, Lammers JW, Koenderman L and Coffer PJ . (2000). Curr. Biol., 10, 1201–1204.
Dong Z and Wang J . (2004). J. Biol. Chem., 279, 9215–9221.
Donze O, Deng J, Curran J, Sladek R, Picard D and Sonerberg N . (2004). EMBO J., 23, 564–571.
Finkel T and Holbrook NJ . (2000). Nature, 408, 239–247.
Gilley J, Coffer PJ and Ham J . (2003). J. Cell Biol., 162, 613–622.
Hockenbery DH, Oltavai ZN, Ying XM, Milliman CL and Korsmeyer SJ . (1993). Cell, 75, 241–251.
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R and Hung MC . (2004). Cell, 117, 225–237.
Huang H-L, Fang L-W, Lu S-P, Chou C-K, Luh T-Y and Lai M-Z . (2003). Oncogene, 22, 8168–8177.
Javelaud D and Besancon F . (2002). J. Biol. Chem., 277, 37949–37954.
Jiang M and Milner J . (2003). Genes Dev., 17, 832–837.
Joshi B, Li L, Taffe BG, Zhu Z, Wahl S, Tian H-S, Ben-Josef E, Taylor JD, Porter AT and Tang DG . (1999). Cancer Res., 59, 4343–4355.
Kinnula VL and Crapo JD . (2003). Am. J. Respir. Crit. Care Med., 167, 1600–1619.
Kops GJPL, Dansen TB, Polderman PE, Saarloos I, Wirtz KWA, Coffer PJ, Huang T-T, Bos JL, Medema RH and Burgering BMT . (2002). Nature, 419, 316–321.
Lin L, Hron JD and Peng SL . (2004). Immunity, 21, 203–213.
Liu JW, Chandra D, Tang SH, Chopra D and Tang DG . (2002). Cancer Res., 62, 2976–2981.
Martin AG and Fearnhead HO . (2002). J. Biol. Chem., 277, 50834–50841.
Micheau O and Tschopp J . (2003). Cell, 114, 181–190.
Mikkelsen RB and Wardman P . (2003). Oncogene, 22, 5734–5754.
Modur V, Nagarajan R, Evers BM and Mildbrandt J . (2002). J. Biol. Chem., 277, 47928–47937.
Nair VD, Warren TYC, Olanow W and Sealfon SC . (2004). J. Biol. Chem., 279, 27494–27501.
Nemoto S and Finkel T . (2002). Science, 295, 2450–2452.
Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F and Wang X . (2003). Genes Dev., 17, 1475–1486.
Plas DR and Thompson CB . (2003). J. Biol. Chem., 278, 12361–12366.
Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A and Johnson EM . (2001). Neuron, 29, 615–628.
Raff MC . (1992). Nature (London), 356, 397–400.
Ramaswamy S, Nakamura N, Sansi I, Bergeron L and Sellers WR . (2002). Cancer Cell, 2, 81–91.
Ricci J-E, Gottlieb RA and Green DR . (2003). J. Cell Biol., 160, 65–75.
Rokudal S, Fujita N, Kitahara O, Nakamura Y and Tsuruo T . (2002). Mol. Cell. Biol., 22, 8695–8708.
Sade H and Sarin A . (2004). Cell Death Differ., 11, 416–423.
Schimidt M, de Mattos SF, van der Horst A, Klompmaker R, Kops GJPL, Lam EW-F, Burgering BMT and Medema RH . (2002). Mol. Cell. Biol., 22, 7842–7852.
Sundaresan M, Yu ZX, Ferrons VJ, Irani K and Finkel T . (1995). Science, 270, 296–299.
Tang DG and Honn KV . (1997). J. Cell. Physiol., 172, 155–170.
Tang DG, Li L, Chopra DP and Porter AT . (1998). Cancer Res., 58, 3466–3479.
Valks DM, Kemp TJ and Clerk A . (2003). J. Biol. Chem., 278, 25542–25547.
Zhao Y, Wang Z-B and Xu J-X . (2003). J. Biol. Chem., 278, 2356–2360.
Acknowledgements
We thank R DePinho for FOXO3a+/+ and FOXO3a−/− MEFs, Biomide Corp. for BMD188, K Claypool for flow analysis, M Raff for advice and critically reading the manuscript, and the Tang lab for support. This work was supported, in part, by NIH Grants CA90297 and AG023374, ACS Grant RSG MGO-105961, DoD Grant DAMD17-03-1-0137, and NIEHS Center Grant 5 P30 ES07784 (all to DGT), and DoD Postdoctoral Fellowships DAMD17-03-1-0146 (J-WL) and DAMD17-02-1-0083 (DC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, JW., Chandra, D., Rudd, M. et al. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene 24, 2020–2031 (2005). https://doi.org/10.1038/sj.onc.1208385
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208385
Keywords
This article is cited by
-
Functional block of IL-17 cytokine promotes bone healing by augmenting FOXO1 and ATF4 activity in cortical bone defect model
Osteoporosis International (2017)
-
The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense
Oncogene (2015)
-
FoxOs: Unifying Links Between Oxidative Stress and Skeletal Homeostasis
Current Osteoporosis Reports (2011)
-
Adenovirus 5 E1A enhances histone deacetylase inhibitors-induced apoptosis through Egr-1-mediated Bim upregulation
Oncogene (2010)
-
Lipocalin-2: pro- or anti-apoptotic?
Cell Biology and Toxicology (2010)