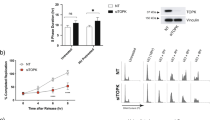
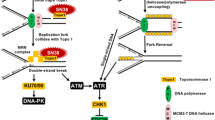
Abstract
Chk1 is the major mediator in the activation of cell-cycle checkpoints in response to a variety of genotoxic stresses. We have previously shown that inhibition of Chk1 sensitizes tumor cells to topoisomerase inhibitors such as camptothecin and doxorubicin through abrogation of cell-cycle arrest (S or G2/M checkpoints). However, it was not clear whether inhibition of Chk1 could potentiate antimetabolites, a mainstay of cancer therapy, which confer genotoxic stress through a different mechanism than topoisomerase inhibitors. 5-Fluorouracil (5-FU) is the most widely used antimetabolite in the treatment of colorectal, breast and other major types of cancers. Here we demonstrate that 5-FU activates Chk1 and induces an early S-phase arrest. Chk1 downregulation abrogates this arrest and dramatically sensitizes tumor cells to the cytotoxic effects of 5-FU. 5-FU confers S-phase arrest through Chk1-mediated Cdc25A proteolysis leading to inhibition of Cdk2. Chk1 elimination stabilizes the Cdc25A protein and results in the abrogation of the S checkpoint and resumption of DNA synthesis, which leads to excessive accumulation of double-stranded DNA breaks. As a result, downregulation of Chk1 potentiates 5-FU efficacy through induction of premature chromosomal condensation followed by apoptosis. Interestingly, the profiles of various cell-cycle markers indicate that cells progress to early M phase to induce apoptosis after checkpoint abrogation. Yet, cells fail to increase their DNA content to 4N as revealed by FACS analysis, probably due to the dramatic induction of double-stranded DNA breaks and chromosomal fragmentation. This is significantly different from the cell-cycle profiles observed in the potentiation of topoisomerase inhibitors by Chk1 siRNA, which showed mitotic progression with 4N DNA content leading to mitotic catastrophe after abrogation of the S or G2 checkpoint. Thus, our results illustrate a novel mode of checkpoint abrogation and cell death conferred by Chk1 inhibition. Additionally, we show that Chk1 deficiency potentiates 5-FU efficacy through the preferential induction of the caspase-8 pathway and subsequent caspase-3 activation. In conclusion, we have clearly demonstrated that inhibition of Chk1 not only potentiates the toxicity of conventional DNA-damaging agents such as ionizing radiation and topoisomerase inhibitors, but also enhances the toxicity of antimetabolites in cancer cell lines. This discovery reveals novel scope of checkpoint abrogation and will significantly broaden the potential application of Chk1 inhibitors in cancer therapy if they do not potentiate the toxicity of 5-FU in normal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Boldin MP, Goncharov TM, Goltsev YV and Wallach D . (1996). Cell, 85, 803–815.
Chen Z, Xiao Z, Chen J, Ng SC, Sowin T, Sham H, Rosenberg S, Fesik S and Zhang H . (2003). Mol. Cancer Ther., 2, 543–548.
Chini CC and Chen J . (2003). J. Biol. Chem., 278, 30057–30062.
Eastman A, Kohn EA, Brown MK, Rathman J, Livingstone M, Blank DH and Gribble GW . (2002). Mol. Cancer Ther., 1, 1067–1078.
Hendry JH and West CML . (1997). Int. J. Radiat. Biol., 71, 709–719.
Johnson DG and Walker CL . (1999). Annu. Rev. Pharmacol. Toxicol., 39, 295–312.
Johnston PG and Kaye S . (2001). Anticancer Drugs, 12, 639–646.
Koniaras K, Cuddihy AR, Christopoulos H, Hogg A and O'Connell MJ . (2001). Oncogene, 20, 7453–7463.
Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA and Elledge SJ . (2001). Genes Dev., 14, 1448–1459.
Lock RB and Stribinskiene L . (1996). Cancer Res., 56, 4006–4012.
Longley DB, Harkin DP and Johnston PG . (2003). Nat. Rev. Cancer, 3, 330–338.
Morgan DO . (1997). Annu. Rev. Cell Dev. Biol., 13, 261–291.
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME and Dixit VM . (1996). Cell, 85, 817–827.
Nghiem P, Park PK, Kim YS, Vazari C and Schreiber SL . (2001). Proc. Natl. Acad. Sci. USA, 98, 9092–9097.
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS and Piwnica-Worms H . (1997). Science, 277, 1501–1505.
Roninson IB, Broude EV and Chang B-D . (2001). Drug Resist. Updates, 4, 303–313.
Sampath D, Rao VA and Plunkett W . (2003). Oncogene, 22, 9063–9074.
Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H and Elledge SJ . (1997). Science, 277, 1497–1501.
Schwartzberg LS, Petak I, Stewart C, Turner PK, Ashley J, Tillman DM, Douglas L, Tan M, Billups C, Mihalik R, Weir A, Tauer K, Shope S and Houghton JA . (2002). Clin. Cancer Res., 8, 2488–2498.
Sedelnikova OA, Pilch DR, Redon C and Bonner WM . (2003). Cancer Biol. Ther., 2, 233–235.
Shi Z, Azuma A, Sampath D, Li YX, Huang P and Plunkett W . (2001). Cancer Res., 61, 1065–1072.
Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J and Lukas J . (2003). Cancer Cell, 3, 247–258.
Strasser A, O'Connor L and Dixit VM . (2000). Annu. Rev. Biochem., 69, 217–245.
Torres K and Horwitz SB . (1998). Cancer Res., 58, 3620–3626.
Tounekti O, Pron G, Belehradek Jr J and Mir LM . (1993). Cancer Res., 53, 5462–5469.
Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S and Zhang H . (2003). J. Biol. Chem., 278, 21767–21773.
Zachos G, Rainey MD and Gillespie DA . (2003). EMBO J., 22, 713–723.
Zhao H, Watkins JL and Piwnica-Worms H . (2002). Proc. Natl. Acad. Sci. USA, 99, 14795–14800.
Zhou BB and Bartek J . (2004). Nat. Rev. Cancer, 4, 216–225.
Acknowledgements
We thank Dr Steve Fesik for his critical reading of the manuscript and for his many stimulating inputs. We also thank Mai Bui and Zehan Chen for help with the FACS analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiao, Z., Xue, J., Sowin, T. et al. A novel mechanism of checkpoint abrogation conferred by Chk1 downregulation. Oncogene 24, 1403–1411 (2005). https://doi.org/10.1038/sj.onc.1208309
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208309
Keywords
This article is cited by
-
Analysis of centrosome and DNA damage response in PLK4 associated Seckel syndrome
European Journal of Human Genetics (2017)
-
Preclinical analyses and phase I evaluation of LY2603618 administered in combination with Pemetrexed and cisplatin in patients with advanced cancer
Investigational New Drugs (2014)
-
Base excision repair AP endonucleases and mismatch repair act together to induce checkpoint-mediated autophagy
Nature Communications (2013)
-
Molecular characterization of apoptosis induced by CARF silencing in human cancer cells
Cell Death & Differentiation (2011)
-
A novel Chk inhibitor, XL-844, increases human cancer cell radiosensitivity through promotion of mitotic catastrophe
Investigational New Drugs (2011)