Abstract
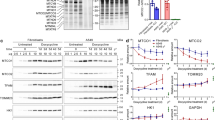
The expression of the tumour suppressor protein fragile histidine triad (Fhit) is often impaired in many human cancers and its restoration in Fhit-negative cancer cell lines suppresses tumorigenicity and induces apoptosis. Although the proapoptotic function of Fhit is well documented, little is known about its precise mechanism of action and further studies are needed in order to elucidate the putative therapeutic properties of this protein. To this end, we have engineered the lung cancer cell line NCI-H460 in order to express different molecules involved in the control of apoptotic pathways. Infection of these cells with an adenoviral vector transducing the Fhit gene (Ad-Fhit) revealed that complete protection from apoptosis was conferred by the inhibitor of caspases Cytokine response modifier A (CrmA) and by a dominant-negative form of the adapter protein Fas-associated death domain (FADD) and partial protection by a dominant-negative form of caspase-8, while cells over expressing mitochondrial mediators of the apoptotic response such as Bcl-2 or Bcl-x(L) that are resistant to treatment with cisplatin, remained highly susceptible to cell death triggered by Fhit gene transfer. In line to what was observed in H460 cells, Ad-Fhit efficacy was not affected by Bcl-2 overexpression also in two other lung cancer cell lines (A549 and Calu-1). Analysis of cytochrome c release also confirmed that in Bcl-2- or Bcl-x(L)-expressing cells apoptosis could be detected by terminal deoxynucleotidyl-transferase mediated dUTP nick-end labelling (TUNEL) assay before any evidence of mitochondrial membrane perturbation. In conclusion, our analysis indicates that the Fhit protein exerts its oncosuppressor activity through induction of an apoptotic mechanism that seems to be FADD dependent, caspase-8 mediated and independent from mitochondrial amplification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- Fhit:
-
fragile histidine triad
- NSLC:
-
non-small-cell lung cancer
- FADD:
-
Fas-associated death domain
- CrmA:
-
cytokine response modifier A
- MOI:
-
multiplicity of infection
- TUNEL:
-
terminal deoxynucleotidyltransferase-mediated dUTP nick-end labelling
References
Baffa R, Gomella LG, Vecchione A, Bassi P, Mimori K, Sedor J, Calviello CM, Gardiman M, Minimo C, Strup SE, McCue PA, Kovatich AJ, Pagano F, Huebner K and Croce CM . (2000). Am. J. Pathol., 156, 419–424.
Broker LE, Huisman C, Ferreira CG, Rodriguez JA, Kruyt FA and Giaccone G . (2002). Cancer Res., 62, 4081–4088.
Cavazzoni A, Petronini PG, Galetti M, Roz L, Andriani F, Carbognani P, Rusca M, Fumarola C, Alfieri R and Sozzi G . (2004). Oncogene, Sep 13 (Epub ahead of print).
Chinnaiyan AM, O'Rourke K, Tewari M and Dixit VM . (1995). Cell, 81, 505–512.
Chinnaiyan AM, O'Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J and Dixit VM . (1996). Science, 274, 990–992.
Connolly DC, Greenspan DL, Wu R, Ren X, Dunn RL, Shah KV, Jones RW, Bosch FX, Munoz N and Cho KR . (2000). Clin. Cancer Res., 6, 3505–3510.
Dumon KR, Ishii H, Fong LY, Zanesi N, Fidanza V, Mancini R, Vecchione A, Baffa R, Trapasso F, During MJ, Huebner K and Croce CM . (2001a). Proc. Natl. Acad. Sci. USA, 98, 3346–3351.
Dumon KR, Ishii H, Vecchione A, Trapasso F, Baldassarre G, Chakrani F, Druck T, Rosato EF, Williams NN, Baffa R, During MJ, Huebner K and Croce CM . (2001b). Cancer Res., 61, 4827–4836.
Ekert PG, Silke J and Vaux DL . (1999). EMBO J., 18, 330–338.
Ferreira CG, Span SW, Peters GJ, Kruyt FA and Giaccone G . (2000). Cancer Res., 60, 7133–7141.
Hengartner MO . (2000). Nature, 407, 770–776.
Huang DC, Cory S and Strasser A . (1997). Oncogene, 14, 405–414.
Huisman C, Ferreira CG, Broker LE, Rodriguez JA, Smit EF, Postmus PE, Kruyt FA and Giaccone G . (2002). Clin. Cancer Res., 8, 596–606.
Ishii H, Dumon KR, Vecchione A, Fong LY, Baffa R, Huebner K and Croce CM . (2001a). JAMA, 286, 2441–2449.
Ishii H, Dumon KR, Vecchione A, Trapasso F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K and Croce CM . (2001b). Cancer Res., 61, 1578–1584.
Ji L, Fang B, Yen N, Fong K, Minna JD and Roth JA . (1999). Cancer Res., 59, 3333–3339.
Johnstone RW, Ruefli AA and Lowe SW . (2002). Cell, 108, 153–164.
Kaufmann SH and Hengartner MO . (2001). Trends Cell Biol., 11, 526–534.
Li H, Zhu H, Xu CJ and Yuan J . (1998). Cell, 94, 491–501.
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X . (1998). Cell, 94, 481–490.
Makin G and Dive C . (2001). Trends Cell Biol., 11, S22–S26.
Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K and Croce CM . (2000). Cancer Res., 60, 1177–1182.
Peto R, Lopez AD, Boreham J, Thun M, Heath Jr C and Doll R . (1996). Br. Med Bull., 52, 12–21.
Roz L, Gramegna M, Ishii H, Croce CM and Sozzi G . (2002). Proc. Natl. Acad. Sci. USA, 99, 3615–3620.
Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo MP, Gramegna M, Croce CM, Pierotti MA and Sozzi G . (1999). Proc. Natl. Acad. Sci. USA, 96, 8489–8492.
Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH and Peter ME . (1999). J. Biol. Chem., 274, 22532–22538.
Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti MA, Croce CM and Pilotti S . (1998). Cancer Res., 58, 5032–5037.
van Heerden WF, Swart TJ, van Heerden MB, van Rensburg EJ, Engelbrecht S, Dreyer L and Huebner K . (1999). J. Oral. Pathol. Med., 28, 433–437.
Zou H, Li Y, Liu X and Wang X . (1999). J Biol. Chem., 274, 11549–11556.
Acknowledgements
We thank Professor P Krammer (Deutsches Krebsforschungszentrum, Heidelberg, Germany) for the anti-caspase-8 antibody, Drs Massimo Zeviani and Valeria Tiranti (Istituto Nazionale Neurologico ‘C Besta’, Milan, Italy) for the anti-COX IV antibody and Drs Delia Mezzanzanica and Paola Perego (Istituto Nazionale Tumori, Milan, Italy) for helpful discussions and for providing the anti-cytochrome c and anti-Bcl-X(L) antibodies, respectively. We are also grateful to Dr Hideshi Ishii for technical advice and Professor Carlo M Croce (both at Kimmel Cancer Center, Jefferson Medical College, Philadelphia, PA, USA) for comments and suggestions. This work was partially supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) and from Ministero Italiano della Sanita' to GS. LR and FA are recipients of fellowships from AIRC and Ministero Italiano della Sanita'.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roz, L., Andriani, F., Ferreira, C. et al. The apoptotic pathway triggered by the Fhit protein in lung cancer cell lines is not affected by Bcl-2 or Bcl-x(L) overexpression. Oncogene 23, 9102–9110 (2004). https://doi.org/10.1038/sj.onc.1208142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208142
Keywords
This article is cited by
-
Germinal epimutation of Fragile Histidine Triad (FHIT) gene is associated with progression to acute and chronic adult T-cell leukemia diseases
Molecular Cancer (2021)
-
Fragile histidine triad protein: structure, function, and its association with tumorogenesis
Journal of Cancer Research and Clinical Oncology (2010)
-
The expression of FHIT, PCNA and EGFR in benign and malignant breast lesions
British Journal of Cancer (2007)
-
High throughput tissue microarray analysis of FHIT expression in diffuse large cell B-cell lymphoma from Saudi Arabia
Modern Pathology (2006)
-
Proapoptotic function of FHIT gene
Chinese Journal of Cancer Research (2006)