Abstract
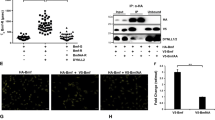
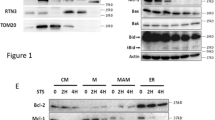
Mcl-1 is an antiapoptotic member of the Bcl-2 family that can promote cell viability. We report here that Mcl-1 is a new substrate for caspases during induction of apoptosis. Mcl-1 cleavage occurs after Asp127 and Asp157 and generates four fragments of 24, 19, 17 and 12 kDa in both intact cells and in vitro, an effect prevented by selective caspase inhibitors. As a consequence, the resulting protein that lacks the first 127 or 157 amino acids contains only the BH1–BH3 domains of Bcl-2 family members. Mutation of Asp127 and Asp157 abolishes the generation of the 24 and 12 kDa fragments and that of the 19 and 17 kDa fragments, respectively. Interestingly, when expressed in HeLa cells Mcl-1 wt and Mcl-1 Δ127 showed a markedly different intracellular distribution. Mcl-1 wt colocalized with α-Tubulin near the internal face of the plasma membrane, while Mcl-1 Δ127 coassociated with Bim-EL at the mitochondrial level. Coimmunoprecipitation experiments also demonstrated that Mcl1 Δ127 exhibited increased binding to Bim when compared to Mcl-1 wt. Finally, Mcl-1 wt unlike Mcl-1 Δ127 inhibited Bim-EL-induced caspase activation. Altogether, our findings demonstrate that cleavage of Mcl-1 by caspases modifies its subcellular localization, increases its association with Bim and inhibits its antiapoptotic function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Akgul C, Moulding DA, White MR and Edwards SW . (2000). FEBS Lett., 478, 72–76.
Bae J, Donigian JR and Hsueh AJ . (2003). J. Biol. Chem., 278, 5195–5204.
Bae J, Leo CP, Hsu SY and Hsueh AJ . (2000). J. Biol. Chem., 275, 25255–25261.
Bertolotto C, Ricci JE, Luciano F, Mari B, Chambard JC and Auberger P . (2000). J. Biol. Chem., 275, 37246–37250.
Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P and Whyte MK . (2000). J. Biol. Chem., 275, 22136–22146.
Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G and Thompson CB . (1993). Cell, 74, 597–608.
Breitschopf K, Haendeler J, Malchow P, Zeiher AM and Dimmeler S . (2000). Mol. Cell. Biol., 20, 1886–1896.
Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K and Hardwick JM . (1997). Science, 278, 1966–1968.
Cory S and Adams JM . (2002). Nat. Rev. Cancer, 2, 647–656.
Craig RW . (2002). Leukemia, 16, 444–454.
Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, Bataille R and Amiot M . (2002). Blood, 100, 194–199.
Domina AM, Smith JH and Craig RW . (2000). J. Biol. Chem., 275, 21688–21694.
Earnshaw WC, Martins LM and Kaufmann SH . (1999). Annu. Rev. Biochem., 68, 383–424.
Fujita N, Nagahashi A, Nagashima K, Rokudai S and Tsuruo T . (1998). Oncogene, 17, 1295–1304.
Han J, Goldstein LA, Gastman BR, Froelich CJ, Yin XM and Rabinowich H . (2004). J. Biol. Chem., 10, 10.
Herrant M, Luciano F, Loubat A and Auberger P . (2002). Oncogene, 21, 4957–4968.
Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, Umezawa A and Ichijo H . (2002). J. Biol. Chem., 277, 43730–43734.
Jacquel A, Herrant M, Legros L, Belhacene N, Luciano F, Pages G, Hofman P and Auberger P . (2003). FASEB J., 4, 4.
Jourdan M, De Vos J, Mechti N and Klein B . (2000). Cell Death Differ., 7, 1244–1252.
Jourdan M, Veyrune JL, Vos JD, Redal N, Couderc G and Klein B . (2003). Oncogene, 22, 2950–2959.
Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD and Reed JC . (1998). Blood, 91, 991–1000.
Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S, Wang HG, Zhang X, Bullrich F, Croce CM, Rai K, Hines J and Reed JC . (1998). Blood, 91, 3379–3389.
Kozopas KM, Yang T, Buchan HL, Zhou P and Craig RW . (1993). Proc. Natl. Acad. Sci. USA, 90, 3516–3520.
Li H, Bergeron L, Cryns V, Pasternack MS, Zhu H, Shi L, Greenberg A and Yuan J . (1997). J. Biol. Chem., 272, 21010–21017.
Luciano F, Herrant M, Jacquel A, Ricci JE and Auberger P . (2003). FASEB J., 17, 711–713.
Luciano F, Ricci JE and Auberger P . (2001). Oncogene, 20, 4935–4941.
Mouhamad S, Besnault L, Auffredou MT, Leprince C, Bourgeade MF, Leca G and Vazquez A . (2004). J. Immunol., 172, 2084–2091.
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC and Korsmeyer SJ . (2003). Nature, 426, 671–676.
Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P and Pouyssegur J . (1999). Science, 286, 1374–1377.
Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, Kipps TJ, Choi YS, Bennett F and Reed JC . (2002). Blood, 100, 1795–1801.
Puthalakath H, Huang DC, O’Reilly LA, King SM and Strasser A . (1999). Mol. Cell, 3, 287–296.
Puthier D, Thabard W, Rapp M, Etrillard M, Harousseau J, Bataille R and Amiot M . (2001). Br. J. Haematol., 112, 358–363.
Reynolds JE, Li J, Craig RW and Eastman A . (1996). Exp. Cell Res., 225, 430–436.
Ricci JE, Lang V, Luciano F, Belhacene N, Giordanengo V, Michel F, Bismuth G and Auberger P . (2001). FASEB J., 15, 1777–1779.
Ricci JE, Maulon L, Luciano F, Guerin S, Livolsi A, Mari B, Breittmayer JP, Peyron JF and Auberger P . (1999). Oncogene, 18, 3963–3969.
Rinkenberger JL, Horning S, Klocke B, Roth K and Korsmeyer SJ . (2000). Genes Dev., 14, 23–27.
Snowden RT, Sun XM, Dyer MJ and Cohen GM . (2003). Leukemia, 17, 1981–1989.
Townsend KJ, Trusty JL, Traupman MA, Eastman A and Craig RW . (1998). Oncogene, 17, 1223–1234.
Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A and Craig RW . (1999). J. Biol. Chem., 274, 1801–1813.
Yang T, Buchan HL, Townsend KJ and Craig RW . (1996). J. Cell Physiol., 166, 523–536.
Zhang B, Gojo I and Fenton RG . (2002). Blood, 99, 1885–1893.
Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, Suzuki H, Mihara K, Kappler J and Marrack P . (2004). Proc. Natl. Acad. Sci. USA, 10, 10.
Zou H, Li Y, Liu X and Wang X . (1999). J. Biol. Chem., 274, 11549–11556.
Acknowledgements
We thank Pr E Solary for the kind gift of the Mcl-1 cDNA. We are indebted to Dr B Mari for critical review of the manuscript. This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Ligue Nationale Contre le Cancer (LNC, Equipe labellisée). Dr S Marchetti and Dr F Luciano are recipients of a LNC fellowship. Imatinib mesylate is a kind gift from Novartis Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrant, M., Jacquel, A., Marchetti, S. et al. Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene 23, 7863–7873 (2004). https://doi.org/10.1038/sj.onc.1208069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208069
Keywords
This article is cited by
-
2D exfoliated black phosphorus influences healthy and cancer prostate cell behaviors
Scientific Reports (2021)
-
MARCH5 requires MTCH2 to coordinate proteasomal turnover of the MCL1:NOXA complex
Cell Death & Differentiation (2020)
-
MARCH5-dependent degradation of MCL1/NOXA complexes defines susceptibility to antimitotic drug treatment
Cell Death & Differentiation (2020)
-
Blockade of HSP70 by VER-155008 synergistically enhances bortezomib-induced cytotoxicity in multiple myeloma
Cell Stress and Chaperones (2020)
-
Hypertonicity-enforced BCL-2 addiction unleashes the cytotoxic potential of death receptors
Oncogene (2018)