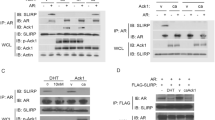
Abstract
The androgen receptor (AR) is a member of the nuclear receptor superfamily of ligand-activated transcription factors and plays a key role in the development and progression of prostate cancer. Current therapies include the use of antiandrogens aimed at inhibiting the transcriptional activation of AR-regulated genes by AR. Here, we explore a strategy aimed at obtaining silencing of AR-regulated genes, based on the properties of the transcriptional repressor promyelocytic leukamia zinc-finger protein (PLZF). In order to do this, we have made a fusion protein between PLZF and AR, named PLZF-AR, and show that PLZF-AR is able to bring about silencing of genomically encoded AR-regulated genes and inhibit the androgen-regulated growth of LNCaP prostate cancer cells. Together, our results show that this strategy is able to bring about potent repression of AR-regulated responses and, therefore, could be of value in the development of new therapies for prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ayer DE . (1999). Trends Cell Biol., 9, 193–198.
Berthois Y, Katzenellenbogen JA and Katzenellenbogen BS . (1986). Proc. Natl. Acad. Sci. USA, 83, 2496–2500.
Bevan CL, Hoare S, Claessens F, Heery DM and Parker MG . (1999). Mol. Cell. Biol., 19, 8383–8392.
Bird AP and Wolffe AP . (1999). Cell, 99, 451–454.
Blankvoort BM, de Groene EM, van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJ and Aarts JM . (2001). Anal. Biochem., 298, 93–102.
Bramlett KS, Dits NF, Sui X, Jorge MC, Zhu X and Jenster G . (2001). Mol. Cell. Endocrinol., 183, 19–28.
Chawla A, Repa JJ, Evans RM and Mangelsdorf DJ . (2001). Science, 294, 1866–1870.
Chen D, Pace PE, Coombes RC and Ali S . (1999). Mol. Cell. Biol., 19, 1002–1015.
Chen SJ, Zelent A, Tong JH, Yu HQ, Wang ZY, Derre J, Berger R, Waxman S and Chen Z . (1993a). J. Clin. Invest., 91, 2260–2267.
Chen Z, Brand NJ, Chen A, Chen SJ, Tong JH, Wang ZY, Waxman S and Zelent A . (1993b). EMBO J., 12, 1161–1167.
Cheung WL, Briggs SD and Allis CD . (2000). Curr. Opin. Cell. Biol., 12, 326–333.
Chung BH, Mitchell SH, Zhang JS and Young CY . (2001). Carcinogenesis, 22, 1201–1206.
Cleutjens KB, van der Korput HA, van Eekelen CC, van Rooij HC, Faber PW and Trapman J . (1997). Mol. Endocrinol., 11, 148–161.
Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO and Trapman J . (1996). J. Biol. Chem., 271, 6379–6388.
Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ and Sugimura Y . (1987). Endocr. Rev., 8, 338–362.
David G, Alland L, Hong SH, Wong CW, DePinho RA and Dejean A . (1998). Oncogene, 16, 2549–2556.
Davies P and Eaton CL . (1991). J. Endocrinol., 131, 5–17.
Dilworth FJ and Chambon P . (2001). Oncogene, 20, 3047–3054.
Feldman BJ and Feldman D . (2001). Nat. Rev. Cancer, 1, 34–45.
Glass CK and Rosenfeld MG . (2000). Genes Dev., 14, 121–141.
Green S, Issemann I and Sheer E . (1988). Nucleic Acids Res., 16, 369.
Greenlee RT, Murray T, Bolden S and Wingo PA . (2000). CA Cancer J. Clin., 50, 7–33.
Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara FF, Zamir I, Seiser C, Lazar MA, Minucci S and Pelicci PG . (1998). Nature, 391, 815–818.
Guidez F, Ivins S, Zhu J, Soderstrom M, Waxman S and Zelent A . (1998). Blood, 91, 2634–2642.
He LZ, Guidez F, Tribioli C, Peruzzi D, Ruthardt M, Zelent A and Pandolfi PP . (1998a). Nat. Genet., 18, 126–135.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW and Vogelstein B . (1998b). Proc. Natl. Acad. Sci. USA, 95, 2509–2514.
Heemers H, Vanderhoydonc F, Heyns W, Verhoeven G and Swinnen JV . (2000). Biochem. Biophys. Res. Commun., 269, 209–212.
Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK and Sandberg AA . (1980). Prog. Clin. Biol. Res., 37, 115–132.
Hsieh ML, Charlesworth MC, Goodmanson M, Zhang S, Seay T, Klee GG, Tindall DJ and Young CY . (1997). Cancer Res., 57, 2651–2656.
Huggins C . (1967). Cancer Res., 27, 1925–1930.
Labrie F, Belanger A, Dupont A, Luu-The V, Simard J and Labrie C . (1993). Clin. Invest. Med., 16, 475–492.
Labrie F, Dupont A, Belanger A, St-Arnaud R, Giguere M, Lacourciere Y, Emond J and Monfette G . (1986). Endocr. Rev., 7, 67–74.
Lin RJ, Nagy L, Inoue S, Shao W, Miller Jr WH and Evans RM . (1998). Nature, 391, 811–814.
Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC and Buluwela L . (1996). Cancer Res., 56, 1155–1163.
Mabjeesh NJ, Zhong H and Simons JW . (2002). Endocr. Relat. Cancer, 9, 115–139.
McKenna NJ and O’Malley BW . (2002). Cell, 108, 465–474.
Mhatre AN, Trifiro MA, Kaufman M, Kazemi-Esfarjani P, Figlewicz D, Rouleau G and Pinsky L . (1993). Nat. Genet., 5, 184–188.
Monne M, Croce CM, Yu H and Diamandis EP . (1994). Cancer Res., 54, 6344–6347.
Montgomery JS, Price DK and Figg WD . (2001). J. Pathol., 195, 138–146.
Narlikar GJ, Fan HY and Kingston RE . (2002). Cell, 108, 475–487.
Razin A and Riggs AD . (1980). Science, 210, 604–610.
Reid A, Gould A, Brand N, Cook M, Strutt P, Li J, Licht J, Waxman S, Krumlauf R and Zelent A . (1995). Blood, 86, 4544–4552.
Roth SY, Denu JM and Allis CD . (2001). Annu. Rev. Biochem., 70, 81–120.
Sadi MV, Walsh PC and Barrack ER . (1991). Cancer, 67, 3057–3064.
Schwabe JW, Chapman L, Finch JT and Rhodes D . (1993). Cell, 75, 567–578.
Shang Y and Brown M . (2002). Science, 295, 2465–2468.
Takane KK and McPhaul MJ . (1996). Mol. Cell. Endocrinol., 119, 83–93.
Tomura A, Goto K, Morinaga H, Nomura M, Okabe T, Yanase T, Takayanagi R and Nawata H . (2001). J. Biol. Chem., 276, 28395–28401.
Tora L, Mullick A, Metzger D, Ponglikitmongkol M, Park I and Chambon P . (1989). EMBO J., 8, 1981–1986.
Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B and Roy AK . (2000). Mol. Endocrinol., 14, 1162–1174.
Ulrix W, Swinnen JV, Heyns W and Verhoeven G . (1999). FEBS Lett, 455, 23–26.
van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W and Trapman J . (1991). Int. J. Cancer, 48, 189–193.
Wong CW and Privalsky ML . (1998). J. Biol. Chem., 273, 27695–27702.
Workman JL and Kingston RE . (1998). Annu. Rev. Biochem., 67, 545–579.
Zegers ND, Claassen E, Neelen C, Mulder E, van Laar JH, Voorhorst MM, Berrevoets CA, Brinkmann AO, van der Kwast TH, Ruizeveld de Winter JA, Trapman J and Boersma WJA . (1991). Biochim. Biophys. Acta, 1073, 23–32.
Zelent A, Guidez F, Melnick A, Waxman S and Licht JD . (2001). Oncogene, 20, 7186–7203.
Zhang Y, LeRoy G, Seelig HP, Lane WS and Reinberg D . (1998). Cell, 95, 279–289.
Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A and Reinberg D . (1999). Genes Dev., 13, 1924–1935.
Zhou ZX, Sar M, Simental JA, Lane MV and Wilson EM . (1994). J. Biol. Chem., 269, 13115–13123.
Acknowledgements
We are grateful to members of the group for continual advice and support. This work was carried out with support from the Charing Cross & Hammersmith Hospitals Trustees, the Prostate Cancer Charity, Cancer Research UK and the Association for International Cancer Research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pike, J., Holmes, D., Kamalati, T. et al. Silencing of androgen-regulated genes using a fusion of AR with the PLZF transcriptional repressor. Oncogene 23, 7561–7570 (2004). https://doi.org/10.1038/sj.onc.1208030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208030
Keywords
This article is cited by
-
The androgen receptor is a tumor suppressor in estrogen receptor–positive breast cancer
Nature Medicine (2021)
-
Equol an isoflavonoid: potential for improved prostate health, in vitro and in vivoevidence
Reproductive Biology and Endocrinology (2011)
-
FBI-1 functions as a novel AR co-repressor in prostate cancer cells
Cellular and Molecular Life Sciences (2011)