Abstract
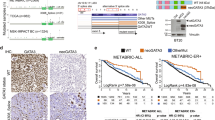
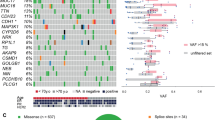
GATA3 is an essential transcription factor that was first identified as a regulator of immune cell function. In recent microarray analyses of human breast tumors, both normal breast luminal epithelium and estrogen receptor (ESR1)-positive tumors showed high expression of GATA3. We sequenced genomic DNA from 111 breast tumors and three breast-tumor-derived cell lines and identified somatic mutations of GATA3 in five tumors and the MCF-7 cell line. These mutations cluster in the vicinity of the highly conserved second zinc-finger that is required for DNA binding. In addition to these five, we identified using cDNA sequencing a unique mis-splicing variant that caused a frameshift mutation. One of the somatic mutations we identified was identical to a germline GATA3 mutation reported in two kindreds with HDR syndrome/OMIM #146255, which is an autosomal dominant syndrome caused by the haplo-insufficiency of GATA3. The ectopic expression of GATA3 in human 293T cells caused the induction of 73 genes including six cytokeratins, and inhibited cell line doubling times. These data suggest that GATA3 is involved in growth control and the maintenance of the differentiated state in epithelial cells, and that GATA3 variants may contribute to tumorigenesis in ESR1-positive breast tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bocker W, Bier B, Freytag G, Brommelkamp B, Jarasch ED, Edel G, Dockhorn-Dworniczak B and Schmid KW . (1992). Virchows Arch. A Pathol. Anat. Histopathol., 421, 315–322.
Boecker W and Buerger H . (2003). Cell Prolif., 36 (Suppl 1), 73–84.
Dairkee SH, Puett L and Hackett AJ . (1988). J. Natl. Cancer Inst., 80, 691–695.
Dennis Jr G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC and Lempicki RA . (2003). Genome Biol., 4, P3.
Finlin BS, Gau CL, Murphy GA, Shao H, Kimel T, Seitz RS, Chiu YF, Botstein D, Brown PO, Der CJ, Tamanoi F, Andres DA and Perou CM . (2001). J. Biol. Chem., 31, 31.
Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC and Srivastava D . (2003). Nature, 424, 443–447.
Graham FL, Smiley J, Russell WC and Nairn R . (1977). J. Gen. Virol., 36, 59–74.
Groet J, McElwaine S, Spinelli M, Rinaldi A, Burtscher I, Mulligan C, Mensah A, Cavani S, Dagna-Bricarelli F, Basso G, Cotter FE and Nizetic D . (2003). Lancet, 361, 1617–1620.
Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A, Ferno M, Peterson C and Meltzer PS . (2001). Cancer Res., 61, 5979–5984.
Guelstein VI, Tchypysheva TA, Ermilova VD, Litvinova LV, Troyanovsky SM and Bannikov GA . (1988). Int. J. Cancer, 42, 147–153.
Ho IC, Vorhees P, Marin N, Oakley BK, Tsai SF, Orkin SH and Leiden JM . (1991). EMBO J., 10, 1187–1192.
Hoch RV, Thompson DA, Baker RJ and Weigel RJ . (1999). Int. J. Cancer, 84, 122–128.
Kaufman CK, Zhou P, Pasolli HA, Rendl M, Bolotin D, Lim KC, Dai X, Alegre ML and Fuchs E . (2003). Genes Dev., 17, 2108–2122.
Kruglyak L and Nickerson DA . (2001). Nat. Genet., 27, 234–236.
Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F and Engel JD . (2000). Nat. Genet., 25, 209–212.
Loots GG, Ovcharenko I, Pachter L, Dubchak I and Rubin EM . (2002). Genome Res., 12, 832–839.
Ma GT, Roth ME, Groskopf JC, Tsai FY, Orkin SH, Grosveld F, Engel JD and Linzer DI . (1997). Development, 124, 907–914.
Morgenstern JP and Land H . (1990). Nucleic Acids Res., 18, 3587–3596.
Muroya K, Hasegawa T, Ito Y, Nagai T, Isotani H, Iwata Y, Yamamoto K, Fujimoto S, Seishu S, Fukushima Y, Hasegawa Y and Ogata T . (2001). J. Med. Genet., 38, 374–380.
Nawijn MC, Ferreira R, Dingjan GM, Kahre O, Drabek D, Karis A, Grosveld F and Hendriks RW . (2001). J. Immunol., 167, 715–723.
Nesbit MA, Bowl MR, Harding B, Ali A, Ayala A, Crowe C, Dobbie A, Hampson G, Holdaway I, Levine MA, McWillliams R, Rigden S, Sampson J, Williams A and Thakker RV . (2004). J. Biol. Chem., 21, 22624–22634.
Orkin SH . (1992). Blood, 80, 575–581.
Packer BR, Yeager M, Staats B, Welch R, Crenshaw A, Kiley M, Eckert A, Beerman M, Miller E, Bergen A, Rothman N, Strausberg R and Chanock SJ . (2004). Nucl. Acids. Res., 32, D528–D532.
Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD and Lindenbaum MH . (1995). Nat. Genet., 11, 40–44.
Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JC, Lashkari D, Shalon D, Brown PO and Botstein D . (1999). Proc. Natl. Acad. Sci. USA, 96, 9212–9217.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO and Botstein D . (2000). Nature, 406, 747–752.
Pollack JR, Sørlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE, Tibshirani R, Botstein D, Borresen-Dale AL and Brown PO . (2002). Proc. Natl. Acad. Sci. USA, 20, 12963–12968.
Ross DT and Perou CM . (2001). Dis. Markers, 17, 99–109.
Sena-Esteves M, Saeki Y, Camp SM, Chiocca EA and Breakefield XO . (1999). J. Virol., 73, 10426–10439.
Smith VM, Lee PP, Szychowski S and Winoto A . (1995). J. Biol. Chem., 270, 1515–1520.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P and Borresen-Dale AL . (2001). Proc. Natl. Acad. Sci. USA, 98, 10869–10874.
Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL and Botstein D . (2003). Proc. Natl. Acad. Sci. USA, 100, 8418–8423.
Tavassoli FA and Schnitt SJ . (1992). Pathology of the Breast. Elsevier: New York.
Troester MA, Lindstrom AB, Waidyanatha S, Kupper LL and Rappaport SM . (2002). Toxicol. Sci., 68, 314–321.
Tusher VG, Tibshirani R and Chu G . (2001). Proc. Natl. Acad. Sci. USA, 98, 5116–5121.
van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, Mross F, Dieterich H, Seitz R, Ross D, Botstein D and Brown P . (2002). Am. J. Pathol., 161, 1991–1996.
Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV and Devriendt K . (2000). Nature, 406, 419–422.
van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R and Friend SH . (2002). Nature, 415, 530–536.
Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM and Crispino JD . (2002). Nat. Genet., 32, 148–152.
West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R, Zuzan H, Olson Jr JA, Marks JR and Nevins JR . (2001). Proc. Natl. Acad. Sci. USA, 98, 11462–11467.
Yang Z, Gu L, Romeo PH, Bories D, Motohashi H, Yamamoto M and Engel JD . (1994). Mol. Cell. Biol., 14, 2201–2212.
Acknowledgements
We wish to thank Jerry Shay (UTSW) for the ME16C cell line. We also thank Phil Bernard (Univ. of Utah) and Juan Palazzo (Thomas Jefferson Univ.) for contributing breast tumor samples to this study. This work was supported by funds from the NCI Breast SPORE program to UNC-CH (C.M.P. P50-CA58223-09A1), The Norwegian Cancer Society (A.-L. B-D D99061), the Research Council of Norway (A.-L. B-D 137012/310 and 155218/30) and the NCI CGAP Project (S.C.). We also wish to acknowledge the technical assistance and support of the Tissue Procurement and Analysis Facility of UNC-CH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Usary, J., Llaca, V., Karaca, G. et al. Mutation of GATA3 in human breast tumors. Oncogene 23, 7669–7678 (2004). https://doi.org/10.1038/sj.onc.1207966
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207966
Keywords
This article is cited by
-
Architecture and topologies of gene regulatory networks associated with breast cancer, adjacent normal, and normal tissues
Functional & Integrative Genomics (2023)
-
GATA3 and MDM2 are synthetic lethal in estrogen receptor-positive breast cancers
Communications Biology (2022)
-
GATA3 Truncating Mutations Promote Cistromic Re-Programming In Vitro, but Not Mammary Tumor Formation in Mice
Journal of Mammary Gland Biology and Neoplasia (2019)
-
A double helical motif in OCIAD2 is essential for its localization, interactions and STAT3 activation
Scientific Reports (2018)
-
Nuclear receptors connect progenitor transcription factors to cell cycle control
Scientific Reports (2017)