Abstract
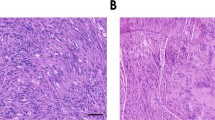
Previously, we have mimicked human neurofibromatosis type 2 (NF2) in conditional Nf2 mutant (P0Cre;Nf2flox2/flox2) mice. Schwannomas, characteristic for NF2, were found at low frequency in older mice. Here, we report that these mice, upon additional hemizygosity for p53, rapidly develop multiple tumours showing features consistent with malignant peripheral nerve sheath tumours. Thus, p53 hemizygosity promotes tumorigenesis of mutant Nf2 peripheral nerve cells. In contrast, young P0Cre;Nf2flox2/+;p53+/− cis mice mainly succumb to Nf2/p53-related osteogenic tumours. Therefore, Cre-mediated early biallelic loss of Nf2 function in neural crest-derived cells hemizygous for p53 results in resistance to osteogenic tumours and increased susceptibility to peripheral nerve sheath tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bianchi AB, Mitsunaga S-I, Cheng JQ, Klein WM, Jhanwar SC, Seizinger B, Kley N, Klein-Szanto AJP and Testa JR . (1995). Proc. Natl. Acad. Sci. USA, 92, 10854–10858.
Bretscher A, Chambers D, Nguyen R and Reczek D . (2000). Annu. Rev. Cell Dev. Biol., 16, 113–143.
Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT and Jacks T . (1999). Science, 286, 2172–2176.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery Jr CA, Butel JS and Bradley A . (1992). Nature, 356, 215–221.
Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery Jr CA, Park SH, Thompson T, Ford RJ and Bradley A . (1995). Mol. Carcinog., 14, 16–22.
Erlandson RA and Woodruff JM . (1982). Cancer, 49, 273–287.
Giovannini M, Robanus-Maandag E, Niwa-Kawakita M, van der Valk M, Woodruff JM, Goutebroze L, Merel P, Berns A and Thomas G . (1999). Genes Dev., 13, 978–986.
Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A and Thomas G . (2000). Genes Dev., 14, 1617–1630.
Harvey M, McArthur MJ, Montgomery Jr CA, Bradley A and Donehower LA . (1993). FASEB J., 7, 938–943.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT and Weinberg RA . (1994). Curr. Biol., 4, 1–7.
Jin JJ, Yu NA and Rajewsky MF . (1993). Cell Growth Differ., 4, 227–237.
Kalamarides M, Niwa-Kawakita M, Leblois H, Abramowski V, Perricaudet M, Janin A, Thomas G, Gutmann DH and Giovannini M . (2002). Genes Dev., 16, 1060–1065.
Kaya G, Rodriguez I, Jorcano JL, Vassalli P and Stamenkovic I . (1997). Genes Dev., 11, 996–1007.
Kleihues P and Sobin LH . (2000). Cancer, 88, 2887.
McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT and Jacks T . (1998). Genes Dev., 12, 1121–1133.
McClatchey AI, Saotome I, Ramesh V, Gusella JF and Jacks T . (1997). Genes Dev., 11, 1253–1265.
McKusick VA . (1994). Mendelian Inheritance in Man: A Catalogue of Human Genes and Genetic Disorders. The John Hopkins University Press: Baltimore & London.
Menon AG, Anderson KM, Riccardi VM, Chung RY, Whaley JM, Yandell DW, Farmer GE, Freiman RN, Lee JK, Li FP, Barker DF, Ledbetter DH, Kleider A, Martuza RL, Gusella JF and Seizinger BR . (1990). Proc. Natl. Acad. Sci. USA, 87, 5435–5439.
Messing A, Behringer RR, Wrabetz L, Hammang JP, Lemke G, Palmiter RD and Brinster RL . (1994). J. Neurosci., 14, 3533–3539.
Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H and Herrlich P . (2001). Genes Dev., 15, 968–980.
Purdie CA, Harrison DJ, Peter A, Dobbie L, White S, Howie SE, Salter DM, Bird CC, Wyllie AH, Hooper ML and Clarke AR . (1994). Oncogene, 9, 603–609.
Rouleau GA, Mérel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, Pulst SM, Lenoir G, Bijlsma E, Fashold R, Dumanski J, de Jong P, Parry D, Eldridge R, Aurias A, Delattre O and Thomas G . (1993). Nature, 363, 515–521.
Sherman L, Sleeman JP, Hennigan RF, Herrlich P and Ratner N . (1999). Oncogene, 18, 6692–6699.
Sherman L, Wainwright D, Ponta H and Herrlich P . (1998). Genes Dev., 12, 1058–1071.
Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, Haase VH, Ambrose CM, Munroe D, Bove C, Haines JL, Martuza RL, MacDonald ME, Seizinger BR, Short MP, Buckler AG and Gusella JF . (1993). Cell, 72, 791–800.
Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ and Parada LF . (1999). Science, 286, 2176–2179.
Zorick TS and Lemke G . (1996). Curr. Opin. Cell Biol., 8, 870–876.
Acknowledgements
We thank L.A. Donehower for the p53−/− mice; F. van der Ahé, K. Ankama, N. Bosnie, T. Maidment, H. Raasø, L. Rijswijk, and A. Zwerver for animal care; the CDTA (Orléans, France) for housing part of the mouse colony; R. Regnerus for genotyping of the mice; J. Bulthuis, K. de Goeij, D. Hoogervorst, L. Kuijper-Pietersma, and E. van Muylwijk for histotechnical assistance; R. Erlandson for electron microscopy; H. te Riele for critically reading the manuscript. This work was supported by Grants from the Commission of the European Communities (BMH4-CT96-1518), Ligue Nationale Française contre le Cancer, Association pour la Recherche sur le Cancer, Human Frontier Science Program (M.G.). United States Army Medical Research and Material Command Grant DAMD 17-00-0594 (M.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robanus-Maandag, E., Giovannini, M., van der Valk, M. et al. Synergy of Nf2 and p53 mutations in development of malignant tumours of neural crest origin. Oncogene 23, 6541–6547 (2004). https://doi.org/10.1038/sj.onc.1207858
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207858
Keywords
This article is cited by
-
Age-dependent copy number variations of TP53 tumour suppressor gene associated with altered phosphorylation status of p53 protein in sporadic schwannomas
Journal of Neuro-Oncology (2019)
-
Search for Chemical Compounds for Pharmacotherapy of Neurofibromatosis Type 2
Pharmaceutical Chemistry Journal (2015)
-
Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes
Oncogene (2011)
-
Treatment of Implantable NF2 Schwannoma Tumor Models with Oncolytic Herpes Simplex Virus G47Δ
Cancer Gene Therapy (2007)