Abstract
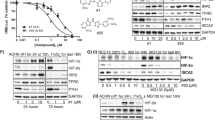
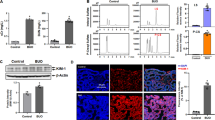
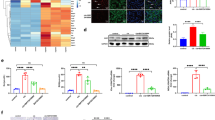
Ferric nitrilotriacetate (Fe-NTA) induces oxidative renal damage leading to a high incidence of renal cell carcinoma (RCC) in rats. Differential display analysis of such RCCs revealed elevated expression of annexin 2 (Anx2), a substrate for kinases and a receptor for tissue-type plasminogen activator and plasminogen. We conducted this study to clarify the significance of Anx2 in Fenton reaction-based carcinogenesis. Messenger RNA and protein levels of Anx2 were increased time-dependently in the rat kidney after Fe-NTA administration as well as in LLC-PK1 cells after exposure to H2O2. The latter was inhibited by pretreatment with N-acetylcysteine, pyrrolidine dithiocarbamate or catalase. Immunohistochemistry revealed negligible staining in the normal renal proximal tubules, but strong staining in regenerating proximal tubules, karyomegalic cells and RCCs. Metastasizing RCCs showed higher Anx2 protein levels. Anx2 was phosphorylated at serine and tyrosine residues in these cells and coimmunoprecipitated with phosphorylated actin. Overexpression of Anx2 induced a higher cell proliferation rate in LLC-PK1 cells. In contrast, a decrease in proliferation leading to apoptosis was observed after Anx2 antisense treatment to cell lines established from Fe-NTA-induced RCCs. These results suggest that Anx2 is regulated by redox status, and that persistent operation of this adaptive mechanism plays a role in the proliferation and metastasis of oxidative stress-induced cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- BrdU:
-
bromodeoxyuridine
- FCS:
-
fetal calf serum
- FDD:
-
fluorescent differential display
- Fe-NTA:
-
ferric nitrilotriacetate
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- HEPES:
-
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
- HNE:
-
4-hydroxy-2-nonenal
- MDA:
-
malondialdehyde
- NAC:
-
N-acetylcysteine
- PBS(-):
-
Dulbecco's phosphate-buffered saline
- PCR:
-
polymerase chain reaction
- PDTC:
-
pyrrolidine dithiocarbamate
- RCC:
-
renal cell carcinoma
- ROS:
-
reactive oxygen species
- RT:
-
reverse transcription
- SDS–PAGE:
-
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- s.e.m.:
-
standard error of the mean
- t-PA:
-
tissue-type plasminogen activator
- Tris:
-
2-amino-2-hydroxymethyl-1,3-propanediol
- TUNEL:
-
terminal deoxynucleotidyl transferase end labeling
References
Aarli A, Skeie Jensen T, Kristoffersen EK, Bakke A and Ulvestad E . (1997). Apmis, 105, 699–704.
Ames BN, Shigenaga MK and Park E-M . (1991). Oxidative Damage and Repair: Chemical, Biological and Medical Aspects, Davies KJA (ed). Pergamon Press: New York, pp. 181–187.
Biener Y, Feinstein R, Mayak M, Kaburagi Y, Kadowaki T and Zick Y . (1996). J. Biol. Chem., 271, 29489–29496.
Chacko G, Ling Q and Hajjar KA . (1998). J. Biol. Chem., 273, 19840–19846.
Chetcuti A, Margan S, Russell P, Mann S, Millar D, Clark S, Rogers J, Handelsman D and Dong Q . (2001). Cancer Res., 61, 6331–6334.
Chiang Y, Schneiderman MH and Vishwanatha JK . (1993). Cancer Res., 53, 6017–6021.
Devary Y, Gottlieb RA, Lau LF and Karin M . (1991). Mol. Cell. Biol., 11, 2804–2811.
Ebina Y, Okada S, Hamazaki S, Ogino F, Li JL and Midorikawa O . (1986). J. Natl. Cancer Inst., 76, 107–113.
Emoto K, Sawada H, Yamada Y, Fujimoto H, Takahama Y, Ueno M, Takayama T, Uchida H, Kamada K, Naito A, Hirao S and Nakajima Y . (2001). Anticancer Res., 21, 1339–1345.
Erikson E, Shealy DJ and Erikson RL . (1981). J. Biol. Chem., 256, 11381–11384.
Forman H, Torres M and Fukuto J . (2002). Mol. Cell. Biochem., 234–235, 49–62.
Frohrich M, Motte P, Galvin K, Takahashi H, Wands J and Ozturk M . (1990). Mol. Cell. Biol., 10, 3216–3223.
Gerke V and Moss S . (2002). Physiol. Rev., 82, 331–371.
Goldman L, Cutrone E, Kotenko S, Krause C and Langer J . (1996). Biotechniques, 21, 1013–1015.
Gould KL, Woodgett JR, Isacke CM and Hunter T . (1986). Mol. Cell. Biol., 6, 2738–2744.
Haigler HT, Schlaepfer DD and Burgess WH . (1987). J. Biol. Chem., 262, 6921–6930.
Hajjar KA and Jacovina AT . (1998). J. Invest. Med., 46, 364–369.
Hajjar KA and Menell JS . (1997). Ann. N.Y. Acad. Sci., 811, 337–349.
Halliwell B and Gutteridge JMC . (1999). Free Radicals in Biology and Medicine, 3rd edn. Clarendon Press: Oxford.
Hamazaki S, Okada S, Ebina Y and Midorikawa O . (1985). Toxicol. Appl. Pharmacol., 77, 267–274.
Hamazaki S, Okada S, Ebina Y, Li JL and Midorikawa O . (1988). Toxicol. Appl. Pharmacol., 92, 500–506.
Hiroyasu M, Ozeki M, Kohda H, Echizenya M, Tanaka T, Hiai H and Toyokuni S . (2002). Am. J. Pathol., 160, 419–424.
Hull R, Cherry W and Weaver G . (1976). In Vitro, 12, 670–677.
Jindal HK, Chaney WG, Anderson CW, Davis RG and Vishwanatha JK . (1991). J. Biol. Chem., 266, 5169–5176.
Johnstone SA, Hubaishy I and Waisman DM . (1992). J. Biol. Chem., 267, 25976–25981.
Jones PG, Moore GJ and Waisman DM . (1992). J. Biol. Chem., 267, 13993–13997.
Konig J, Prenen J, Nilius B and Gerke V . (1998). J. Biol. Chem., 273, 19679–19684.
Krimpenfort P, Quon K, Mooi W, Loonstra A and Berns A . (2001). Nature, 413, 83–86.
Kumble KD, Hirota M, Pour PM and Vishwanatha JK . (1992a). Cancer Res., 52, 163–167.
Kumble KD, Iversen PL and Vishwanatha JK . (1992b). J. Cell Sci., 101, 35–41.
Langen R, Isas JM, Hubbell WL and Haigler HT . (1998). Proc. Natl. Acad. Sci. USA, 95, 14060–14065.
Li JL, Okada S, Hamazaki S, Ebina Y and Midorikawa O . (1987). Cancer Res., 47, 1867–1869.
Li N and Karin M . (1999). FASEB J., 13, 1137–1143.
Liu L, Wang M, Fisher AB and Zimmerman UJ . (1996). Am. J. Physiol., 270, L668–676.
Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA and Hajjar KA . (1999). N. Engl. J. Med., 340, 994–1004.
Mignatti P, Robbins E and Rifkin DB . (1986). Cell, 47, 487–498.
Nakanishi H, Takeuchi S, Kato K, Shimizu S, Kobayashi K, Tatematsu M and Shirai T . (1996). Jpn. J. Cancer Res., 87, 1218–1226.
Nishiyama Y, Suwa H, Okamoto K, Fukumoto M, Hiai H and Toyokuni S . (1995). Jpn. J. Cancer Res., 86, 1150–1158.
Nose K, Shibanuba M, Kikuchi K, Kageyama H, Sakiyama S and Kuroki T . (1991). Eur. J. Biochem., 201, 99–106.
Ozaki T and Sakiyama S . (1993). Oncogene, 8, 1707–1710.
Paciucci R, Tora M, Diaz VM and Real FX . (1998). Oncogene, 16, 625–633.
Radke K and Martin GS . (1979). Proc. Natl. Acad. Sci. USA, 76, 5212–5216.
Radke K, Gilmore T and Martin GS . (1980). Cell, 21, 821–828.
Ramasamy S, Parthasarathy S and Harrison DG . (1998). J. Lipid Res., 39, 268–276.
Reeves SA, Chavez-Kappel C, Davis R, Rosenblum M and Israel MA . (1992). Cancer Res., 52, 6871–6876.
Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D and De Pinho R . (1996). Cell, 85, 27–37.
Sharpless N, Bardeesy N, Lee K, Carrasco D, Castrillon D, Aguirre A, Wu E, Horner J and De Pinho R . (2001). Nature, 413, 86–91.
Sun Y and Oberley LW . (1996). Free Radic. Biol. Med., 21, 335–348.
Tanaka T, Iwasa Y, Kondo S, Hiai H and Toyokuni S . (1999). Oncogene, 18, 3793–3797.
Tanaka T, Kondo S, Iwasa Y, Hiai H and Toyokuni S . (2000). Am. J. Pathol., 156, 2149–2157.
Tanaka T, Nishiyama Y, Okada K, Hirota K, Matsui M, Yodoi J, Hiai H and Toyokuni S . (1997). Lab. Invest., 77, 145–155.
Toyokuni S . (1999). Pathol. Int., 49, 91–102.
Toyokuni S, Kawaguchi W, Akatsuka S, Hiroyasu M and Hiai H . (2003). Pathol. Int., 53, 259–261.
Toyokuni S, Luo XP, Tanaka T, Uchida K, Hiai H and Lehotay DC . (1997a). Free Radic. Biol. Med, 22, 1019–1027.
Toyokuni S, Mori T and Dizdaroglu M . (1994a). Int. J. Cancer, 57, 123–128.
Toyokuni S, Mori T, Hiai H and Dizdaroglu M . (1995a). Int. J. Cancer, 62, 309–313.
Toyokuni S, Okada S, Hamazaki S, Minamiyama Y, Yamada Y, Liang P, Fukunaga Y and Midorikawa O . (1990). Cancer Res., 50, 5574–5580.
Toyokuni S, Okamoto K, Yodoi J and Hiai H . (1995b). FEBS Lett., 358, 1–3.
Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Ochi H, Hiai H, Uchida K and Osawa T . (1997b). Lab. Invest., 76, 365–374.
Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H and Stadtman ER . (1994b). Proc. Natl. Acad. Sci. USA, 91, 2616–2620.
Wu T, Angus C, Yao X, Logun C and Shelhamer J . (1997). J. Biol. Chem., 272, 17145–17153.
Zhang D, Okada S, Yu Y, Zheng P, Yamaguchi R and Kasai H . (1997). Cancer Res., 57, 2410–2414.
Zhao W, Chen G, Chen H, Pascale A, Ravindranath L, Quon M and Alkon D . (2003). J. Biol. Chem., 278, 4205–4215.
Zhou S, Kachhap S and Singh KK . (2003). Mutagenesis, 18, 287–292.
Acknowledgements
We thank Dr Jerome A Langer (Rutgers University, Piscataway, New Jersey) for providing pcDEF3 expression vector, and Ms Waka Kawaguchi for excellent technical assistance. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan, a grant from the Program for Promotion of Basic Research Activities for Innovative Bioscience (PROBRAIN) and the Eiko Yasuhara Memorial Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, T., Akatsuka, S., Ozeki, M. et al. Redox regulation of annexin 2 and its implications for oxidative stress-induced renal carcinogenesis and metastasis. Oncogene 23, 3980–3989 (2004). https://doi.org/10.1038/sj.onc.1207555
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207555
Keywords
This article is cited by
-
Receptor role of the annexin A2 in the mesothelial endocytosis of crocidolite fibers
Laboratory Investigation (2015)
-
Human epididymis protein 4 in association with Annexin II promotes invasion and metastasis of ovarian cancer cells
Molecular Cancer (2014)
-
Annexin A2 participates in human skin keloid formation by inhibiting fibroblast proliferation
Archives of Dermatological Research (2014)
-
Prognostic significance of annexin II expression in non-small cell lung cancer
Clinical and Translational Oncology (2013)
-
Quantitative proteomic analysis identifying three annexins as lymph node metastasis-related proteins in lung adenocarcinoma
Medical Oncology (2012)