Abstract
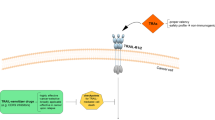
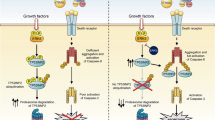
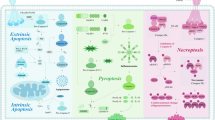
Tumor necrosis factor-related apoptosis-inducing ligand or Apo 2 ligand (TRAIL/Apo2L) is a member of the tumor necrosis factor (TNF) family of ligands capable of initiating apoptosis through engagement of its death receptors. TRAIL selectively induces apoptosis of a variety of tumor cells and transformed cells, but not most normal cells, and therefore has garnered intense interest as a promising agent for cancer therapy. TRAIL is expressed on different cells of the immune system and plays a role in both T-cell- and natural killer cell-mediated tumor surveillance and suppression of suppressing tumor metastasis. Some mismatch-repair-deficient tumors evade TRAIL-induced apoptosis and acquire TRAIL resistance through different mechanisms. Death receptors, members of the TNF receptor family, signal apoptosis independently of the p53 tumor-suppressor gene. TRAIL treatment in combination with chemo- or radiotherapy enhances TRAIL sensitivity or reverses TRAIL resistance by regulating the downstream effectors. Efforts to identify agents that activate death receptors or block specific effectors may improve therapeutic design. In this review, we summarize recent insights into the apoptosis-signaling pathways stimulated by TRAIL, present our current understanding of the physiological role of this ligand and the potential of its application for cancer therapy and prevention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Abdollahi T, Robertson NM, Abdollahi A and Litwack G . (2003). Cancer Res., 63, 4521–4526.
Adams JM and Cory S . (1998). Science, 281, 1322–1326.
Armeanu S, Lauer UM, Smirnow I, Schenk M, Weiss TS, Gregor M and Bitzer M . (2003). Cancer Res., 63, 2369–2372.
Ashkenazi A . (2002). Nat. Rev. Cancer, 2, 420–430.
Ashkenazi A and Dixit VM . (1998). Science, 281, 1305–1308.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z and Schwall RH . (1999). J. Clin. Invest., 104, 155–162.
Banerjee D . (2001). Curr. Opin. Investig. Drugs, 2, 574–580.
Bodmer JL, Meier P, Tschopp J and Schneider P . (2000). J. Biol. Chem., 275, 20632–20637.
Burns TF and El-Deiry WS . (2001). J. Biol. Chem., 276, 37879–37886.
Bykov VJ, Issaeva N, Selivanova G and Wiman KG . (2002). Carcinogenesis, 23, 2011–2018.
Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME and Yang X . (2002). EMBO J., 21, 3704–3714.
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J and Hood L . (1997). Immunity, 7, 821–830.
Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ and Smyth MJ . (2002). J. Immunol., 168, 1356–1361.
Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA and Goodwin RG . (1997). Immunity, 7, 813–820.
Deng Y, Lin Y and Wu X . (2002). Genes Dev., 16, 33–45.
Du C, Fang M, Li Y, Li L and Wang X . (2000). Cell, 102, 33–42.
Eggert A, Grotzer MA, Zuzak TJ, Wiewrodt BR, Ho R, Ikegaki N and Brodeur GM . (2001). Cancer Res., 61, 1314–1319.
El-Deiry WS . (2001). Cell Death Differ., 8, 1066–1075.
Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC and Young PR . (1998). J. Biol. Chem., 273, 14363–14367.
Foster BA, Coffey HA, Morin MJ and Rastinejad F . (1999). Science, 286, 2507–2510.
Frese S, Pirnia F, Miescher D, Krajewski S, Borner MM, Reed JC and Schmid RA . (2003). Oncogene, 22, 5427–5435.
Fulda S, Meyer E, Friesen C, Susin SA, Kroemer G and Debatin KM . (2001). Oncogene, 20, 1063–1075.
Fulda S, Wick W, Weller M and Debatin KM . (2002). Nat. Med., 8, 808–815.
Green DR . (2000). Cell, 102, 1–4.
Griffith TS, Chin WA, Jackson GC, Lynch DH and Kubin ME. (1998). J. Immunol., 161, 2833–2840.
Gura T . (1997). Science, 277, 768.
Harper N, Hughes MA, Farrow SN, Cohen GM and MacFarlane M . (2003). J. Biol. Chem., (in press).
Hersey P and Zhang XD . (2001). Nat. Rev. Cancer, 1, 142–150.
Holen I, Croucher PI, Hamdy FC and Eaton CL . (2002). Cancer Res., 62, 1619–1623.
Hunt A and Evan G . (2001). Science, 293, 1784–1785.
Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'Connell M, Kelley RF, Ashkenazi A and de Vos AM . (1999). Mol. Cell, 4, 563–571.
Ivanov VN, Bhoumik A and Ronai Z . (2003). Oncogene, 22, 3152–3161.
Jansen B, Schlagbauer-Wadl H, Brown BD, Bryan RN, van Elsas A, Muller M, Wolff K, Eichler HG and Pehamberger H . (1998). Nat. Med., 4, 232–234.
Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR and Strom SC . (2000). Nat. Med., 6, 564–567.
Johnson TR, Stone K, Nikrad M, Yeh T, Zong WX, Thompson CB, Nesterov A and Kraft AS . (2003). Oncogene, 22, 4953–4963.
Kim K, Fisher MJ, Xu SQ and El-Deiry WS . (2000). Clin. Cancer Res., 6, 335–346.
Kim Y, Suh N, Sporn M and Reed JC . (2002). J. Biol. Chem., 277, 22320–22329.
Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J and Chen YH . (2003). Nat. Immunol., 4, 255–260.
LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and Ashkenazi A . (2002). Nat. Med., 8, 274–281.
LeBlanc HN and Ashkenazi A . (2003). Cell Death Differ., 10, 66–75.
Lee J, Hampl M, Albert P and Fine HA . (2002). Neoplasia, 4, 312–323.
Lin T, Huang X, Gu J, Zhang L, Roth JA, Xiong M, Curley SA, Yu Y, Hunt KK and Fang B . (2002). Oncogene, 21, 8020–8028.
Mariani SM and Krammer PH . (1998). Eur. J. Immunol., 28, 1492–1498.
Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P and Ashkenazi A . (1997). Curr. Biol., 7, 1003–1006.
McCormick F . (2001). Nat. Rev. Cancer., 1, 130–141.
Mongkolsapaya J, Grimes JM, Chen N, Xu XN, Stuart DI, Jones EY and Screaton GR . (1999). Nat. Struct. Biol., 6, 1048–1053.
Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, Pineiro A, Larrad L, Alava MA, Nava J and Anel A . (2001). J. Immunol., 167, 6736–6744.
Nagane M, Huang HJ and Cavenee WK . (2001). Apoptosis, 6, 191–197.
Ozoren N and El-Deiry W . (2003). Semin. Cancer Biol., 13, 135–147.
Ozoren N and El-Deiry WS . (2002). Neoplasia, 4, 551–557.
Ozoren N, Kim K, Burns TF, Dicker DT, Moscioni AD and El-Deiry WS . (2000). Cancer Res., 60, 6259–6265.
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J and Dixit VM . (1997a). Science, 276, 111–113.
Pan G, Ni J, Wei YF, Yu G, Gentz R and Dixit VM . (1997b). Science, 277, 815–818.
Ravi R and Bedi A . (2002). Cancer Res., 62, 1583–1587.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME . (1998). EMBO J., 17, 1675–1687.
Schmaltz C, Alpdogan O, Kappel BJ, Muriglan SJ, Rotolo JA, Ongchin J, Willis LM, Greenberg AS, Eng JM, Crawford JM, Murphy GF, Yagita H, Walczak H, Peschon JJ and van den Brink MR . (2002). Nat. Med., 8, 1433–1437.
Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ and Sayers TJ . (2003). Cancer Res., 63, 207–213.
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P and Ashkenazi A . (1997). Science, 277, 818–821.
Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR and Yagita H . (2003). Immunity, 18, 1–6.
Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C and Lowe SW . (2001). Nature, 409, 207–211.
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H and Okumura K . (2001). Nat. Med., 7, 94–100.
Takimoto R, Wang W, Dicker DT, Rastinejad F, Lyssikatos J and El-Deiry WS . (2002). Cancer Biol. Ther., 1, 47–55.
Teicher BA, Ara G, Herbst R, Palombella VJ and Adams J . (1999). Clin. Cancer Res., 5, 2638–2645.
Thakkar H, Chen X, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N and Srivastava RK . (2001). J. Biol. Chem., 276, 38361–38369.
Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, McLaughlin MM, Srinivasula SM, Livi GP, Marshall LA, Alnemri ES, Williams WV and Doyle ML . (2000). J. Biol. Chem., 275, 23319–23325.
Tschopp J, Irmler M and Thome M . (1998). Curr. Opin. Immunol., 10, 552–558.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL . (2000). Cell, 102, 43–53.
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG and Rauch CT . (1997). EMBO J., 16, 5386–5397.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith CA, Smolak P, Goodwin RG, Rauch CT, Schuh JC and Lynch DH . (1999). Nat. Med., 5, 157–163.
Wang S and El-Deiry . (2003). Proc. Natl. Acad. Sci. USA., (in press).
Wang W, Takimoto R, Rastinejad F and El-Deiry WS . (2003). Mol. Cell. Biol., 23, 2171–2181.
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA and Goodwin RG . (1995). Immunity, 3, 673–682.
Wu GS, Burns TF, McDonald III ER, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G and El-Deiry WS . (1997). Nat. Genet., 17, 141–143.
Zhang XD, Franco A, Myers K, Gray C, Nguyen T and Hersey P . (1999). Cancer Res., 59, 2747–2753.
Zhang XD, Zhang XY, Gray CP, Nguyen T and Hersey P . (2001). Cancer Res., 61, 7339–7348.
Acknowledgements
WS El-Deiry is an Assistant Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., El-Deiry, W. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 22, 8628–8633 (2003). https://doi.org/10.1038/sj.onc.1207232
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207232
Keywords
This article is cited by
-
The potential role of hydrogen sulfide in cancer cell apoptosis
Cell Death Discovery (2024)
-
p53 stabilisation potentiates [177Lu]Lu-DOTATATE treatment in neuroblastoma xenografts
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
IL-1β stimulated human umbilical cord mesenchymal stem cells ameliorate rheumatoid arthritis via inducing apoptosis of fibroblast-like synoviocytes
Scientific Reports (2023)
-
The effects of IL-1β stimulated human umbilical cord mesenchymal stem cells on polarization and apoptosis of macrophages in rheumatoid arthritis
Scientific Reports (2023)
-
Carboxypeptidase A4 negatively regulates HGS-ETR1/2-induced pyroptosis by forming a positive feedback loop with the AKT signalling pathway
Cell Death & Disease (2023)