Abstract
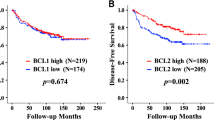
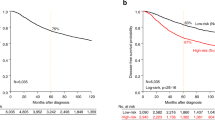
Recently, cyclin-E was reported to be the most prominent prognostic factor for breast cancer outcome described so far, even surpassing axillary nodal involvement. Earlier studies on the prognostic value of cyclin-E in breast cancer, however, yielded heterogeneous results. Therefore, we set out to confirm and extend these results by quantitative Taqman RT–PCR of cyclin-E levels in 277 resectable breast cancers. Cyclin-E levels were not associated with relapse-free survival (RFS) or overall survival (OS) in the total cohort of patients, or in the subset of patients without involved lymph nodes that were not treated with adjuvant systemic therapy. Besides several classical clinicopathological factors, the interaction between cyclin-E and adjuvant endocrine therapy (P=0.01, HR=3.04, 95% CI: 1.30–7.09) was found to contribute significantly in multivariate analyses. Cyclin-E levels were associated with poor RFS specifically in patients treated with adjuvant endocrine therapy (n=108, P=0.001, HR=4.01, 95% CI: 1.76–9.12), independent of estrogen receptor status. In conclusion, cyclin-E is not a pure prognostic factor in breast cancer, but rather a predictor of failure of endocrine therapy. Differences in literature on the presumed prognostic value of cyclin-E may be due to differences in treatment. Assessment of cyclin-E levels can aid in improving adjuvant treatment selection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Altman DG, Lausen B, Sauerbrei W and Schumacher M . (1994). J. Natl. Cancer Inst., 86, 829–835.
Altman DG and Lyman GH . (1998). Breast Cancer Res. Treat., 52, 289–303.
Bindels EM, Lallemand F, Balkenende A, Verwoerd D and Michalides R . (2002). Oncogene, 21, 8158–8165.
Cox DR . (1972). J. R. Stat. Soc. B, 34, 187–220.
Dhillon NK and Mudryj M . (2002). Oncogene, 21, 4626–4634.
Donnellan R, Kleinschmidt I and Chetty R . (2001). Hum. Pathol., 32, 89–94.
Dulic V, Lees E and Reed SI . (1992). Science, 257, 1958–1961.
Foekens JA, Ries C, Look MP, Gippner-Steppert C, Klijn JG and Jochum M . (2003). Cancer Res., 63, 337–341.
Harbeck N, Kates RE, Look MP, Meijer-Van Gelder ME, Klijn JG, Kruger A, Kiechle M, Janicke F, Schmitt M and Foekens JA . (2002). Cancer Res., 62, 4617–4622.
Kaplan EL and Meier P . (1958). J. Am. Statist. Assoc., 53, 457–481.
Keyomarsi K, Conte Jr D, Toyofuku W and Fox MP . (1995). Oncogene, 11, 941–950.
Keyomarsi K and Herliczek TW . (1997). Prog. Cell Cycle Res., 3, 171–191.
Keyomarsi K and Pardee AB . (1993). Proc. Natl. Acad. Sci. USA, 90, 1112–1116.
Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, Bedrosian I, Knickerbocker C, Toyofuku W, Lowe M, Herliczek TW and Bacus SS . (2002). N. Engl. J. Med., 347, 1566–1575.
Kim HK, Park IA, Heo DS, Noh DY, Choe KJ, Bang YJ and Kim NK . (2001). Eur. J. Surg. Oncol., 27, 464–471.
Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR and Roberts JM . (1992). Science, 257, 1689–1694.
Kühling H, Alm P, Olsson H, Ferno M, Baldetorp B, Parwaresch R and Rudolph P . (2003). J. Pathol., 199, 424–431.
Mu"ller-Tidow C, Metzger R, Kugler K, Diederichs S, Idos G, Thomas M, Dockhorn-Dworniczak B, Schneider PM, Koeffler HP, Berdel WE and Serve H . (2001). Cancer Res., 61, 647–653.
Nielsen NH, Arnerlov C, Emdin SO and Landberg G . (1996). Br. J. Cancer, 74, 874–880.
Nielsen NH, Emdin SO, Cajander J and Landberg G . (1997). Oncogene, 14, 295–304.
Payton M, Scully S, Chung G and Coats S . (2002). Oncogene, 21, 8529–8534.
Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR and Roberts JM . (1997). Nat. Med., 3, 222–225.
Porter DC, Zhang N, Danes C, McGahren MJ, Harwell RM, Faruki S and Keyomarsi K . (2001). Mol. Cell. Biol., 21, 6254–6269.
Spruck CH, Won KA and Reed SI . (1999). Nature, 401, 297–300.
Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O and Reed SI . (2001). Nature, 413, 316–322.
Acknowledgements
We thank the contributors, especially the surgeons and internists, of the University Medical Center Nijmegen, Nijmegen; Ziekenhuis Apeldoorn, Apeldoorn; Deventer Ziekenhuis, Deventer; Nieuw Spitaal, Zutphen; and Streekziekenhuis Zevenaar, Zevenaar, The Netherlands, for their assistance in collecting the patients' clinical follow-up data. Doorlène van Tienoven and Anneke Geurts-Moespot are acknowledged for their work with collecting and archiving the breast tumor samples. Joop Heuvel is greatly acknowledged for his expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Span, P., Tjan-Heijnen, V., Manders, P. et al. Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene 22, 4898–4904 (2003). https://doi.org/10.1038/sj.onc.1206818
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206818
Keywords
This article is cited by
-
NUDT22 promotes cancer growth through pyrimidine salvage
Oncogene (2023)
-
Prognostic value of cyclin E expression in breast cancer: a meta-analysis
Tumor Biology (2013)
-
Can predictive biomarkers in breast cancer guide adjuvant endocrine therapy?
Nature Reviews Clinical Oncology (2012)
-
Roscovitine confers tumor suppressive effect on therapy-resistant breast tumor cells
Breast Cancer Research (2011)
-
Biological determinants of endocrine resistance in breast cancer
Nature Reviews Cancer (2009)