Abstract
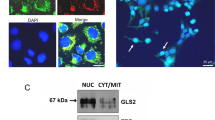
Porphobilinogen deaminase (PBGD) is a rate-limiting enzyme of the heme biosynthesis pathway, whose level is elevated in various human tumors. PBGD was observed in both nuclear and cytoplasmic fractions of C6 glioma cells by immunostaining. During mitosis, chromatids were intensely stained for PBGD in comparison to the interphase chromatin. Using the yeast two-hybrid system, we identified RanBPM, the nuclear Ran-binding protein, as an interacting partner of PBGD. During butyrate-induced differentiation of C6, both nuclear and cytoplasmic PBGD levels declined as did Ran protein and its nucleotide exchange factor RCC1. N,N′-hexamethylene bis-acetamide-dependent differentiation resulted in an increase of the cytoplasmic PBGD, whereas nuclear PBGD, Ran protein and RCC1 remained unchanged. mRNA levels of PBGD remained unchanged during stimulation with both butyrate and N,N′-hexamethylene bis-acetamide. The enzymatic activity of PBGD and protoporphyrin IX synthesis in C6 cells were dependent on the differentiation induction agent. We conclude that PBGD possibly has a nuclear role in addition to its cytosolic enzymatic activity required for heme synthesis, which is related to cell transformation and differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Annunziato AT, Frado LL, Seale RL and Woodcock CL . (1988). Chromosoma, 96, 132–138.
Arcangeli A, Carla M, del Bene MR, Becchetti A, Wanke E and Olivotto M . (1993). Proc. Natl. Acad. Sci. USA, 90, 5858–5862.
Arcangeli A, Ricupero L and Olivotto M . (1987). J. Cell Physiol., 132, 387–400.
Archer SY and Hodin RA . (1999). Curr. Opin. Genet. Dev., 9, 171–174.
Breslow R, Jursic B, Fa Yan Z, Friedman E, Leng L, Ngo L, Rifkind RA and Marks PA . (1991). Proc. Natl. Acad. Sci. USA, 88, 5542–5546.
Cheung WL, Briggs SD and Allis CD . (2000). Curr. Opin. Cell Biol., 12, 326–333.
Chuanmao Z, Hughes M and Clarke PR . (1999). J. Cell Sci., 112, 2453–2461.
Dot J, Danchev N, Blanco I and Rodriguez-Alvarez J . (2002). Neuroreport, 13, 1083–1087.
Gibson SL, Cupriks DJ, Havens JJ, Nguyen ML and Hilf R . (1998). Br. J. Cancer, 77, 235–243.
Goya L, Feng PT, Aliabadi S and Timiras PS . (1996). Int. J. Dev. Neurosci., 14, 409–417.
Greenbaum L, Gozlan Y, Schwartz D, Katcoff DJ and Malik Z . (2002). Br. J. Cancer, 86, 1006–1011.
Hinnen P, de Rooij FW, van Velthuysen ML, Edixhoven A, van Hillegersberg R, Tilanus HW, Wilson JH and Siersema PD . (1998). Br. J. Cancer, 78, 679–682.
Ideguchi H, Ueda A, Tanaka M, Yang J, Tsuji T, Ohno S, Hagiwara E, Aoki A and Ishigatsubo Y . (2002). Biochem. J., 367, 87–95.
Izaurralde E, Kutay U, von Kobbe C, Mattaj IW and Gorlich D . (1997). EMBO J., 16, 6535–6547.
Kennedy JC, Pottier RH and Pross DC . (1990). J. Photochem. Photobiol. B:Biol., 6, 143–148.
Kriegmair M, Baumgartner R, Knuchel R, Stepp H, Hofstadter F and Hofstadter A . (1996). J. Urol., 155, 105–110.
Kuersten S, Ohno M and Mattaj LW . (2001). Trends Cell Biol., 11, 497–503.
Louie GV, Brownlie PD, Lambert R, Cooper JB, Blundell TL, Wood SP, Warren MJ, Woodcock SC and Jordan PM . (1992). Nature, 359, 33–39.
Malik Z, Kostenich G, Rothmann C, Barshack I and Orenstein A . (1998). Lasers Med. Sci., 13, 112–118.
Malik Z and Lugaci H . (1987). Br. J. Cancer, 56, 589–595.
Marks PA, Richon VM, Kiyokawa H and Rifkind RA . (1994). Proc. Natl. Acad. Sci. USA, 91, 10251–10254.
Marks PA, Sheffery M and Rifkind RA . (1987). Cancer Res., 47, 659–666.
McLintock LA and Fitzsimons EJ . (2002). Hematology, 7, 189–195.
Nemergut ME, Mizzen CA, Stukenberg T, Allis CD and Macara IG . (2001). Science, 292, 1540–1543.
Nishitani H, Hirose E, Uchimura Y, Nakamura M, Umeda M, Nishii K, Mori N and Nishimoto T . (2001). Gene, 272, 25–33.
Orenstein A, Haik J, Tamir J, Winkler E, Trau H, Malik Z and Kostenich G . (2000). Dermatol. Surg., 26, 765–770.
Orenstein A, Kostenich G, Kogan L and Malik Z . (1995). Cancer Lett., 93, 227–232.
Orenstein A, Kostenich G, Tsur H, Katanick D, Kopolovic J, Ehrenberg B, Roitman L and Malik Z . (1996). Lasers Life Sci., 7, 1–9.
Ortel B, Chen N, Brissette J, Dotto GP, Maytin E and Hasan T . (1998). Br. J. Cancer, 77, 1744–1751.
Parker KP, Norenberg MD and Vernadakis . (1980). Science, 208, 179–181.
Peng Q, Berg K, Moan J, Kongshaug M and Nesland JM . (1997a). Photochem. Photobiol., 65, 235–251.
Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE and Nesland JM . (1997b). Cancer, 79, 2282–2308.
Ponka P . (1997). Blood, 89, 1–25.
Quimby BB and Corbett AH . (2001). Cell Mol. Life Sci., 58, 1766–1773.
Rifkind RA, Richon VM and Marks PA . (1996). Pharmacol. Ther., 69, 97–102.
Sazer S and Dasso M . (2000). J. Cell Sci., 113, 1111–1118.
Schoenfeld N, Mamet R, Leibovici L, Epstein O, Teitz Y and Atsmon A . (1988). Biochem. Med. Metab. Biol., 40, 213–217.
Segovia J, Lawless JM, Tillakaratne NJK, Brenner M and Tobin A . (1994). J. Neurochem., 63, 1218–1225.
Stepp H, Sroka R and Baumgartner R . (1998). Endoscopy, 30, 379–386.
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R and Reulen HJ . (1998). Neurosurgery, 42, 518–526.
Tunstall RG, Barnett AA, Schofield J, Griffiths J, Vernon DI, Brown SB and Roberts DJH . (2002). Br. J. Cancer, 87, 246–250.
Wang D, Li Z, Messing EM and Wu G . (2002). J. Biol. Chem., 277, 36216–36222.
Yoneda Y . (2000). Genes to Cells, 5, 777–787.
Acknowledgements
We thank HemeBiotech, Denmark for the generous gift of anti-human PBGD antibodies. We are grateful to Dr Takeharu Nishimoto, Kyushu University, Japan for the generous gift of anti-human RanBPM antibodies. Thanks to Ms Judith Hanania for her skilful help during the execution of this study. This study was supported by a GIF Grant no. 052-202.08/98.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greenbaum, L., Katcoff, D., Dou, H. et al. A porphobilinogen deaminase (PBGD) Ran-binding protein interaction is implicated in nuclear trafficking of PBGD in differentiating glioma cells. Oncogene 22, 5221–5228 (2003). https://doi.org/10.1038/sj.onc.1206723
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206723
Keywords
This article is cited by
-
Smad6 is a protein kinase X phosphorylation substrate and is required for HL-60 cell differentiation
Oncogene (2006)
-
From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome
Nature Reviews Neuroscience (2005)
-
Differentiation-dependent photodynamic therapy regulated by porphobilinogen deaminase in B16 melanoma
British Journal of Cancer (2004)