Abstract
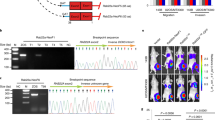
Neuroblastoma (Nb) is a malignancy of the sympathetic nervous system which affects children in their first decade. It is the most common extra-cranial solid tumor in children with an incidence of approximately 1 in 8–10 000 live births annually and accounts for approximately 10% of all children's cancers. Ganglioneuroblastoma is a relatively benign form of Nb and consists of a mixture of fibrils, mature and maturing ganglion cells, as well as undifferentiated neuroblasts. During routine cytogenetic analysis of patients with different manifestations of neuroblastoma we have identified one patient with ganglioneuroblastoma that carries an apparently balanced t(1:13)(q21:q12) reciprocal translocation. Positional cloning of the translocation breakpoint on chromosome 13 resulted in the mapping of the breakpoint between coding exon 2 and exon 3 of WAVE3, a member of WASP gene family. Although the breakpoint region on chromosome 1 was localized to within 2 kb of genomic sequence, no gene was found to be interrupted on this chromosome. The WAVE3 transcript is mainly expressed in the nervous system and, like all the members of the WASP gene family, WAVE3 is a key element in actin polymerization and cytoskeleton organization. WAVE3, therefore, is important for cell differentiation and motility and its expression is lost in a number of low grade and stage 4S tumors. From analysis of its expression pattern and function, WAVE3 is a candidate tumor suppressor gene, at least in some forms of neuroblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ . 1990 J. Mol. Biol. 215: 403–410
Anderson J, Kempski H, Hill L, Rampling D, Gordon T, Michalski A . 2001 Br. J. Cancer 85: 493–496
Burge C, Karlin S . 1997 J. Mol. Biol. 268: 78–94
Castleberry RP . 1999 J. Pediatr. Hematol. Oncol. 21: 178–180
Chernova OB, Hunyadi A, Malaj E, Pan H, Crooks C, Roe B, Cowell JK . 2001 Oncogene 20: 5378–5392
Chernova OB, Somerville RP, Cowell JK . 1998 Oncogene 17: 2873–2881
D'Angio GJ, Evans AE, Koop CE . 1971 Lancet 1: 1046–1049
Fountain JW, Wallace MR, Bruce MA, Seizinger BR, Menon AG, Gusella JF, Michels VV, Schmidt MA, Dewald GW, Collins FS . 1989 Science 244: 1085–1087
Fryns JP . 1996 Genet. Couns. 7: 73
Hirai M, Yoshida S, Kashiwagi H, Kawamura T, Ishikawa T, Kaneko M, Ohkawa H, Nakagawara A, Miwa M, Uchida K . 1999 Genes Chromosomes Cancer 25: 261–269
Kitamura E, Su G, Alaoui-Soussy K, Malaj E, Lewis J, Pan H, Hawthorn LA, Roe B, Cowell JK . 2000 Oncogene 19: 5772–5780
Knudson AG . 1971 Proc. Natl. Acad. Sci. USA 68: 820–823
Knudson AG, Strong LC . 1972 Am. J. Hum. Genet. 24: 514–532
Kushner BH, Gilbert F, Helson L . 1986 Cancer 57: 1887–1893
Lanciotti M, Montaldo PG, Folghera S, Lucarelli E, Cornaglia-Ferraris P, Ponzoni M . 1992 Cell. Mol. Neurobiol. 12: 225–240
Laureys G, Speleman F, Opdenakker G, Benoit Y, Leroy J . 1990 Genes Chromosomes Cancer 2: 252–254
Mead RS, Cowell JK . 1995 Cancer Genet. Cytogenet. 81: 151–157
Michalski AJ, Cotter FE, Cowell JK . 1992 Oncogene 7: 1595–1602
Michalski AJ, Cowell JK . 1993 Genomics 18: 141–143
Miki H, Suetsugu S, Takenawa T . 1998 EMBO J. 17: 6932–6941
Miki H, Yamaguchi H, Suetsugu S, Takenawa T . 2000 Nature 408: 732–735
Mitchell CD . 1991 Br. Med. Bull. 47: 136–156
Mitchell CD, Cowell JK . 1989 Oncogene 4: 253–257
Mora J, Cheung NK, Kushner BH, LaQuaglia MP, Kramer K, Fazzari M, Heller G, Chen L, Gerald WL . 2000 J. Mol. Diagn. 2: 37–46
Nagano H, Kano Y, Kobuchi S, Kajitani T . 1980 Jinrui Idengaku Zasshi 25: 39–45
O'Connell P, Leach R, Cawthon RM, Culver M, Stevens J, Viskochil D, Fournier RE, Rich DC, Ledbetter DH, White R . 1989 Science 244: 1087–1088
Ponzoni M, Lanciotti M, Montaldo PG, Cornaglia-Ferraris P . 1991 Cell. Mol. Neurobiol. 11: 397–413
Riccardi VM, Sujasnsky E, Smith AC, Francke U . 1978 Pediatrics 61: 604–610
Roberts T, Chernova O, Cowell JK . 1998a Cancer Genet. Cytogenet. 100: 10–20
Roberts T, Chernova O, Cowell JK . 1998b Hum. Mol. Genet. 7: 1169–1178
Sambrook J, Fritsch EF, Maniatis T . 1989 Molecular cloning: a laboratory manual 2nd edn. Nolan C (ed) Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press
Sanger WG, Howe J, Fordyce R, Purtilo DT . 1984 Cancer Genet. Cytogenet. 11: 153–159
Sossey-Alaoui K, Kitamura E, Cowell JK . 2001 Int. J. Mol. Med. 7: 543–546
Sossey-Alaoui K, Lyon JA, Jones L, Abidi FE, Hartung AJ, Hane B, Schwartz CE, Stevenson RE, Srivastava AK . 1999 Genomics 60: 330–340
Still IH, Cowell JK . 1998 Blood 92: 1456–1458
Suetsugu S, Miki H, Takenawa T . 1999 Biochem. Biophys. Res. Commun. 260: 296–302
Takenawa T, Miki H . 2001 J. Cell. Sci. 114: 1801–1809
Van Roy N, Laureys G, Van Gele M, Opdenakker G, Miura R, van der Drift P, Chan A, Versteeg R, Speleman F . 1997 Eur. J. Cancer 33: 1974–1978
Varesco L, Thomas HJ, Cottrell S, Murday V, Fennell SJ, Williams S, Searle S, Sheer D, Bodmer WF, Frischauf AM, Soloman E . 1989 Proc. Natl. Acad. Sci. USA 86: 10118–10122
Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD . 2000 EMBO J 19: 4589–4600
Xu Y, Einstein JR, Mural RJ, Shah M, Uberbacher EC . 1994 Proc. Int. Conf. Intell. Syst. Mol. Biol. 2: 376–384
Yunis JJ, Ramsay N . 1978 Am. J. Dis. Child 132: 161–163
Acknowledgements
The authors wish to acknowledge NIH grant NS35791 which supported this work as well as the Roswell Park Cancer Center Support Grant (P30 CA 16056–26). We are grateful to the Children's Oncology Group for providing tumor samples for this study. We would also like to thank Eiko Kitamura, Ph.D. and Karen Head, MS and Lisa Wylie for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sossey-Alaoui, K., Su, G., Malaj, E. et al. WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene 21, 5967–5974 (2002). https://doi.org/10.1038/sj.onc.1205734
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205734
Keywords
This article is cited by
-
Endocytosis in cancer and cancer therapy
Nature Reviews Cancer (2023)
-
Phosphorylation of the proline-rich domain of WAVE3 drives its oncogenic activity in breast cancer
Scientific Reports (2021)
-
WAVE3 phosphorylation regulates the interplay between PI3K, TGF-β, and EGF signaling pathways in breast cancer
Oncogenesis (2020)
-
The STAT3 inhibitor pyrimethamine displays anti-cancer and immune stimulatory effects in murine models of breast cancer
Cancer Immunology, Immunotherapy (2018)
-
WASF3 provides the conduit to facilitate invasion and metastasis in breast cancer cells through HER2/HER3 signaling
Oncogene (2016)