Abstract
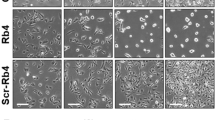
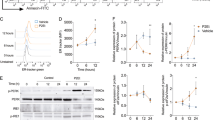
Ceramide has been proposed as a second messenger for stress-induced apoptosis. By characterization of murine melanoma cells and their E1A transfectants, we found several lines of evidences against the role of ceramide as a second messenger for ultraviolet (UV)-induced apoptosis. First, although E1A transfected melanoma cells were more sensitive to UV-induced apoptosis than parental cells, the relative endogenous ceramide elevation induced by UV was greater in parental cells than in E1A transfectants. Second, UV-resistant melanoma cells were more sensitive to exogenous ceramide than UV-sensitive E1A transfectants. The differential responses to UV and ceramide by E1A require the same functional CR2 domain of E1A. Third, unlike the action of UV, transient exposure (up to 2 h) of lethal dose of ceramide was not sufficient to cause apoptosis in these cells, and persistent presence of ceramide was required for processing the apoptotic process. Finally, ceramide and UV do not share a common pathway in apoptosis induction. UV-induced apoptosis was blocked by interleukin-1β-converting enzyme (ICE) inhibitor z-VAD whereas ceramide-induced apoptosis was not. Therefore, we conclude that ceramide is not a general second messenger for UV-induced apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R . 1995 Cell 82: 405–414
Chen Y-R, Wang X, Templeton D, Davis RJ, Tan TH . 1996 J. Biol. Chem. 271: 31929–31936
Cheng A, Caffrey M . 1996 Biophysics 70: 2212–2222
Dbaibo GS, Pushkareva MY, Jayadev S, Schwarz JK, Horowitz JM, Obeid LM, Hannun YA . 1995 Proc. Natl. Acad. Sci. USA 92: 1347–1351
Deng J, Xia W, Hung M-C . 1998 Oncogene 17: 2167–2175
Devary Y, Gottlieb RA, Smeal T, Karin M . 1992 Cell 71: 1081–1091
Dressler KA, Kolesnick RN . 1990 J. Biol. Chem. 265: 14917–14921
Eckner R, Ewen ME, Newsome D, Gerdes M, DeCaprio JA, Lawrence JB, Livingston DM . 1994 Genes Dev. 8: 869–884
Egan C, Bayley ST, Branton PE . 1989 Oncogene 4: 383–338
Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, Kolesnick RN . 1994 J. Exp. Med. 180: 525–535
Hannun YA . 1996 Science 274: 1855–1859
Hannun YA, Obeid LM . 1995 Trends Biochem. Sci. 20: 73–77
Hus SC, Wu CC, Luh TY, Chu CK, Han SH, Lai MZ . 1998 Blood 91: 2658–2663
Karasavvas N, Zaker Z . 1999 Cell Death Differ. 6: 115–123
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG . 1993 Cancer Res. 53: 3976–3985
Kolesnick RN, Haimovitz-Friedmen A, Fuks Z . 1994 Biochem. Cell Biol. 72: 471–474
Lowe SW, Ruley HE . 1993 Genes Dev. 7: 535–545
Martin SJ, Green DR . 1995 Cell 82: 349–352
Martin SJ, Newmeyer DD, Mathias S, Farschon DM, Wang H-G, Reed JC, Kolesnick RN, Green DR . 1995 EMBO J. 14: 5191–5200
McConkey DJ, Goodrich D, Bucana C, Klostergaard J . 1996 Oncogene 13: 1693–1700
Mymryk JS, Shire K, Bayley ST . 1994 Oncogene 9: 1187–1193
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C . 1991 J. Immunol. Methods 139: 271–279
Pena LA, Fuks Z, Kolesnick R . 1997 Biochem. Pharmacol. 53: 615–621
Pierceall WE, Kripke ML, Ananthaswamy HN . 1992 Cancer Res. 52: 3946–3951
Sabbatini P, Lin J, Levine AJ, White E . 1995 Genes Dev. 9: 2184–2192
Shao R, Hu MC, Zhou BP, Lin SY, Chiao PJ, von Lindern RH, Spohn B, Hung M-C . 1998 J. Biol. Chem. 274: 21495–21498
Shao R, Karunagaran D, Zhou BP, Li K, Lo SS, Deng J, Chiao P, Hung MC . 1997 J. Biol. Chem. 272: 32739–32742
Shisler J, Duerksen-Hughes P, Hermiston TM, Wold WSM, Gooding LR . 1996 J. Virol. 70: 68–77
Sillence D, Allan D . 1997 Biochem. J. 324: 29–32
Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MH, Szabo E, Zon LI, Kyriakis JM, Hainovitz-Friedman A, Fuks Z, Kolesnick RN . 1996 Nature 380: 75–79
Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA . 1995 J. Biol. Chem. 270: 22689–22692
White E . 1996 Genes Dev. 10: 1–15
Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E . 1988 Nature 334: 124–129
Wylli AH . 1980 Int. Rev. Cytol. 68: 251–306
Acknowledgements
We thank Dr Stanley T Bayley for providing the mutant E1A plasmid constructs. This research was supported by grants R01 CA58880, CA77858, and cancer center core grant 16672 from the National Cancer Institute; MD Anderson Faculty Achievement Award; and the Breast Cancer Research Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, J., Zhang, H., Kloosterboer, F. et al. Ceramide does not act as a general second messenger for ultraviolet-induced apoptosis. Oncogene 21, 44–52 (2002). https://doi.org/10.1038/sj.onc.1204900
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204900
Keywords
This article is cited by
-
Sphingolipids and mitochondrial apoptosis
Journal of Bioenergetics and Biomembranes (2016)
-
Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin
Oncogene (2004)