Abstract
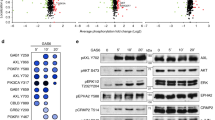
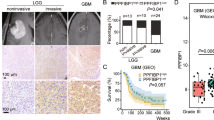
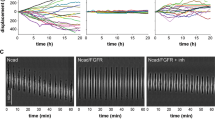
Protein kinase C (PKC) is a family of serine/threonine kinases involved in the transduction of a variety of signals. There is increasing evidence to indicate that specific PKC isoforms are involved in the regulation of distinct cellular processes. In glioma cells, PKC α was found to be a critical regulator of proliferation and cell cycle progression, while PKC ε was found to regulate adhesion and migration. Herein, we report that specific PKC isoforms are able to differentially activate extracellular-signal regulated kinase (ERK) in distinct cellular locations: while PKC α induces the activation of nuclear ERK, PKC ε induces the activation of ERK at focal adhesions. Inhibition of the ERK pathway completely abolished the PKC-induced integrin-mediated adhesion and migration. Thus, we present the first evidence that PKC ε is able to activate ERK at focal adhesions to mediate glioma cell adhesion and motility, providing a molecular mechanism to explain the different biological functions of PKC α and ε in glioma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Besson A, Yong VW . 2000 Mol. Cell. Biol. 20: 4580–4590
Berens ME, Giese A . 1999 Neoplasia 1: 208–219
El-Obeid A, Bongcam-Rudloff E, Sorby M, Ostman A, Nister M, Westermark B . 1997 Cancer Res. 57: 5598–5604
El-Shemerly MYM, Besser D, Nagasawa M, Nagamine Y . 1997 J. Biol. Chem. 272: 30599–30602
Fincham VJ, James M, Frame MC, Winder SJ . 2000 EMBO J. 19: 2911–2923
Friedlander DR, Zagzag D, Shiff B, Cohen H, Allen JC, Kelly PJ, Grumet M . 1996 Cancer Res. 56: 1939–1947
Garrington TP, Johnson GL . 1999 Curr. Opin. Cell Biol. 11: 211–218
Giancotti FG, Ruoslahti E . 1999 Science 285: 1028–1032
Gillespie GY, Soroceanu L, Manning TJ, Gladson CL, Rosenfeld SS . 1999 Cancer Res. 59: 2076–2082
Holland EC . 2000 Proc. Natl. Acad. Sci. USA 97: 6242–6244
Howe AK, Juliano RL . 1998 J. Biol. Chem. 273: 27268–27274
Khoshyomm S, Penar PL, Rossi J, Wells A, Abramson DL, Bhushan A . 1999 Neurosurgery 44: 568–578
Klemke RL, Cai S, Giannini AL, Gallagher PJ, De Lanerolle P, Cheresch DA . 1997 J. Cell Biol. 137: 481–492
Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P . 1999 EMBO J. 18: 2459–2471
Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, Laerum OD . 1990 Cancer Res. 50: 6039–6044
Miranti CK, Ohno S, Brugge JS . 1999 J. Biol. Chem. 274: 10571–10581
Newton AC . 1997 Curr. Opin. Cell Biol. 9: 161–167
Nguyen DHD, Catling AD, Webb DJ, Sankovic M, Walker LA, Somlyo AV, Weber MK, Gonias SL . 1999 J. Cell. Biol. 146: 149–164
Rigot V, Lehmann M, Andre F, Daemi N, Marvaldi J, Luis J . 1998 J. Cell Sci. 111: 3119–3127
Schaeffer HJ, Weber MJ . 1999 Mol. Cell. Biol. 19: 2435–2444
Schonwasser DC, Marais RM, Marshall CJ, Parker PJ . 1998 Mol. Cell. Biol. 18: 790–798
Short SM, Boyer JL, Juliano RL . 2000 J. Biol. Chem. 275: 12970–12977
Sieg DJ, Hauck CR, Schlaepfer DD . 1999 J. Cell Sci. 112: 2677–2691
Traub O, Monia BP, Dean NM, Berk BC . 1997 J. Biol. Chem. 272: 31251–31257
Tysnes OB, Laerum OD . 1993 Anticancer Res. 13: 1325–1330
Zhang W, Law RE, Hinton DR, Couldwell WT . 1997 Cancer Lett. 120: 31–38
Acknowledgements
We gratefully thank Tammy L Wilson for technical assistance in the adhesion assays. This work is supported by a grant from the Canadian Institutes for Health Research. A Besson is a Research Student of the National Cancer Institute of Canada supported with funds provided by the Terry Fox Run. A Davy is the recipient of an Alberta Cancer Board postdoctoral fellowship. SM Robbins is a scholar of the Alberta Heritage Foundation for Medical Research and holds a Canada Research Chair in Cancer Biology. VW Yong is a Medical Research Council of Canada Scientist and a senior scholar of the Alberta Heritage Foundation for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Besson, A., Davy, A., Robbins, S. et al. Differential activation of ERKs to focal adhesions by PKC ε is required for PMA-induced adhesion and migration of human glioma cells. Oncogene 20, 7398–7407 (2001). https://doi.org/10.1038/sj.onc.1204899
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204899
Keywords
This article is cited by
-
From signalling pathways to targeted therapies: unravelling glioblastoma’s secrets and harnessing two decades of progress
Signal Transduction and Targeted Therapy (2023)
-
ZINC40099027 promotes monolayer circular defect closure by a novel pathway involving cytosolic activation of focal adhesion kinase and downstream paxillin and ERK1/2
Cell and Tissue Research (2022)
-
Therapeutic Potential of Berberine in the Treatment of Glioma: Insights into Its Regulatory Mechanisms
Cellular and Molecular Neurobiology (2021)
-
The limitations of targeting MEK signalling in Glioblastoma therapy
Scientific Reports (2020)
-
Brain-Derived Neurotrophic Factor Induces Cell Survival and the Migration of Murine Adult Hippocampal Precursor Cells During Differentiation In Vitro
Neurotoxicity Research (2017)