Abstract
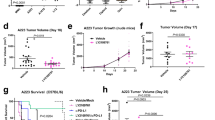
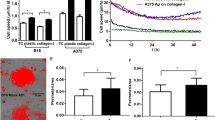
Growth Regulated Oncogene-α (GRO-α) is an autocrine growth factor in melanoma and is a member of the C-X-C family of chemokines which promote chemotaxis of granulocytes and endothelia through binding to CXC Receptor 2. We found previously that variants of murine squamous cell carcinoma PAM 212 which grow and metastasize more rapidly in vivo constitutively express increased levels of murine GRO-α, designated mGRO-α, or KC. We have examined the possible role of mGRO-α expression in malignant progression of squamous cell carcinoma PAM 212 in homologous BALB/c and BALB CXC Receptor-2 deficient mice. Transfection of the PAM 212 cell line which exhibits low expression of GRO-α and malignant potential with a pActin-KC vector encoding mGRO-α enabled isolation of PAM-KC expressing cell lines. These PAM-KC transfectants displayed an increased rate of growth and metastasis in BALB/c mice, similar to the highly malignant phenotype observed in spontaneously occurring metastatic variants. Furthermore, the PAM-KC tumors showed an increase in infiltration of host leukocytes and CD31+ blood vessels, consistent with increased CXC chemokine activity. The increased growth of PAM-KC cells was attenuated in CXCR-2 deficient mice, indicating that the increased growth was dependent in part upon host cells responsive to the CXC chemokine. Together, these results show that a CXC chemokine such as GRO-α can promote malignant growth of murine squamous cell carcinoma by a host CXCR-2 dependent pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Balentien E, Mufson BE, Shattuck RL, Derynck R and Richmond A. . 1991 Oncogene 6: 1115–1124.
Bozie CR, Kolakowski LF, Gerard NP, Garcia-Rodriquez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ and Gerard C. . 1995 J. Immunol. 154: 6048–6057.
Chen Z, Colon I, Ortiz N, Callister M, Pegram M, Arosarena O, Strome S, Nicholson JC and Van Waes C. . 1998 Cancer Res. 58: 3668–3676.
Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith C, Kroog GS, Enamorado I, McCullagh L, Rudy S, Yeh NT, Mousa S, Quezado M, Herscher LL and Van Waes C. . 1999 Clin. Cancer Res. 5: 1369–1379.
Chen Z, Smith CW, Kiel D and Van Waes C. . 1997 Clin. Exp. Metastasis 15: 527–537.
Cohen R, Contrino J, Spiro J, Mann E, Chen L and Kreutzer D. . 1995 Arch. Otolaryngol. Head Neck Surg. 121: 202–209.
Dong G, Chen Z, Kato T and Van Waes C. . 1999 Cancer Res. 59: 3495–3504.
Dong G, Loukinova E, Smith CW, Chen Z and Van Waes C. . 1997 J. Cell. Biochem., (Suppl) 28/29: 90–100.
Duffey D, Chen Z, Dong G, Ondrey FG, Wolf JS, Brown K, Siebenlist U and Van Waes C. . 1999 Cancer Res. 59: 3468–3474.
Gasparini G, Weidner N, Maluta S, Pozza F, Boracchi P, Mezzetti M, Testolin A and Bevilacqua P. . 1993 Int. J. Cancer 55: 739–744.
Gutman M, Singh RK, Yoon S, Xie K, Bucana CD and Fidler JJ. . 1994 Cancer Biother. 9: 163–170.
Moore BB, Arenberg DA, Stoy K, Morgan T, Addison CL, Morris SB, Glass M, Wilke C, Xue YY, Sitterding S, Kunkel SL, Burdick MD and Strieter RM. . 1999 Am. J. Pathol. 154: 1503–1512.
Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N and Van Waes C. . 1999 Mol. Carcinog. 26: 119–129.
Oquendo P, Alberta J, Wen D, Graycar JL, Derynck R and Stiles CD. . 1989 J. Biol. Chem. 264: 4133–4137.
Pak AS, Wright MA, Matthews JP, Collins SL, Petruzelli GJ and Young MRI. . 1995 Clin. Cancer Res. 1: 95–103.
Pekarek L, Starr B, Toledano A and Schreiber H. . 1995 J. Exp. Med. 181: 435–440.
Schreiber H and Rowley DA. . 1999 Inflammation and Cancer: Inflammation: Basic principles and clinical correlates, 3rd edn, Gallin JI and Snyderman R. (eds). Lippincott Williams & Wilkins: Philadelphia pp. 1117–1129.
Seung L, Seung S and Schreiber H. . 1995 Cancer Res. 55: 5094–5100.
Smith CW, Chen Z, Dong G, Loukinova E, Pegram MY, Nicholas-Figueroa L and Van Waes C. . 1998 Clin. Exp. Metastasis 16: 655–664.
Smith D, Polverini P, Kunkel S, Orringer M, Whyte R, Burdick M, Wilke C and Strieter R. . 1994 J. Exp. Med. 179: 1409–1415.
Strieter RM, Kunkel SL, Shanafelt AB, Arenberg DA, Koch AE and Polverini PJ. . 1996 C-X-C chemokines in regulation of angiogenesis: Chemokines in Disease. Koch AE and Strieter RM. (eds). Austin TX: RG Landis pp.195–210.
Van Waes C, Chen Z, Callister M, Colon I, Ortiz N, Thomas GR and Dong G. . 2000 New Frontiers in Immunobiology Veldman JE, Passali D and Lim DJ. (eds). Kugler Publications: The Hague, Netherlands in press.
Weidner N and Folkman J. . 1996 Important Adv. Oncol. 1996: 167–190.
Young MRI, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL and Petruzelli GJ. . 1997 Int. J. Cancer 74: 69–74.
Yuspa SH, Hawley-Nelson P, Koehler B and Stanley JR. . 1980 Cancer Res. 40: 4694–4703.
Acknowledgements
We thank Drs James Battey and Stuart Yuspa for helpful discussions during the course of these studies and comments on the manuscript. The study was supported by NIDCD intramural research project Z01-DC-00016 (C Van Waes) and NCI grant CA-22677 (H Schreiber).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loukinova, E., Dong, G., Enamorado-Ayalya, I. et al. Growth Regulated Oncogene-α expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC Receptor-2 dependent mechanism. Oncogene 19, 3477–3486 (2000). https://doi.org/10.1038/sj.onc.1203687
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203687
Keywords
This article is cited by
-
Targeting oral tumor microenvironment for effective therapy
Cancer Cell International (2023)
-
Curcumin and chemokines: mechanism of action and therapeutic potential in inflammatory diseases
Inflammopharmacology (2023)
-
Tumor regionalization after surgery: Roles of the tumor microenvironment and neutrophil extracellular traps
Experimental & Molecular Medicine (2022)
-
Crosstalk between cancer cells and endothelial cells: implications for tumor progression and intervention
Archives of Pharmacal Research (2018)
-
Thrombocytosis as a prognostic factor in inflammatory breast cancer
Breast Cancer Research and Treatment (2017)