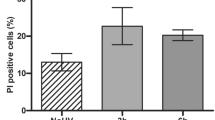
Abstract
The epidermis is continually exposed to harmful mutagens that have the potential to cause DNA damage. To protect the skin from accumulating mutated cells, keratinocytes have developed a highly regulated mechanism of eliminating damaged cells through apoptosis. Bcl-xL is a well-described cell survival protein that when overexpressed in skin can protect keratinocytes from UV radiation-induced apoptosis. To begin to unravel the complex mechanisms that keratinocytes use to survive, we wanted to characterize the role of endogenous Bcl-xL in protecting cells from death. In this study, we describe the development and characterization of an antisense inhibitor to Bcl-xL. We show that this inhibitor reduces Bcl-xL RNA and protein in a concentration-dependent, sequence-specific manner. Furthermore, treatment of keratinocytes and epithelial cells with this inhibitor sensitizes these cells to UV-B radiation and cisplatinum treatment-induced apoptosis. Thus, these results offer direct evidence that Bcl-xL is critical in the protection of skin and epithelial cells from apoptosis and provide a basis for the role of Bcl-xL in keratinocyte and epithelial cell survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ackermann EJ, Taylor JK, Narayana R and Bennett CF. . 1999 J. Biol. Chem. 274: 11245–11252.
Adams JM and Cory S. . 1998 Science 281: 1322–1326.
Allsopp TE, Wyatt S, Paterson HF and Davies AM. . 1993 Cell 73: 295–307.
Altmann K-H, Fabbro D, Dean NM, Geiger T, Monia BP, Muller M and Nicklin P. . 1996 Biochem. Soc. Trans. 24: 630–637.
Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DEH, Witte ON and Green DR. . 1998 Oncogene 16: 1383–1390.
Bennett CF, Condon T, Grimm S, Chan H and Chiang M-Y. . 1994 J. Immunol. 152: 3530–3540.
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Braden HP, Halperin AJ and Ponten J. . 1991 Proc Natl. Acad. Sci. USA 88: 10124–10128.
Carson DA and Lois A. . 1995 Lancet 346: 1009–1011.
Condon TP and Bennett CF. . 1996 J. Biol. Chem. 271: 398–403.
Cotton J and Spandau DF. . 1997 Radiat. Res. 147: 148–155.
Cox LS and Lane DP. . 1995 BioEssays 17: 501–508.
Dassonneville L and Bailly C. . 1998 Bull. Cancer 85: 254–261.
Dean NM and Griffey RH. . 1997 Antisense Nucleic Drug Dev. 7: 229–233.
Dean NM, McKay R, Condon TP and Bennett CF. . 1994 J. Biol. Chem. 269: 16416–16424.
Dean NM, McKay R, Miraglia L, Howard R, Cooper S, Giddings J, Nicklin P, Meister L, Zeil R, Geiger T, Muller M and Fabbro D. . 1996 Cancer Res. 56: 3499–3507.
Deng G and Podack ER. . 1993 Proc. Natl. Acad. Sci. USA 90: 2189–2193.
Dhanwada KR, Dickens M, Neades R, Davis R and Pelling JC. . 1995 Oncogene 11: 1947–1953.
Fujio Y, Kunisada K, Hirota H, Yamauchi-Takihara K and Kishimoto T. . 1997 J. Clin. Invest. 99: 2898–2905.
Gniadecki R, Hansen M and Wulf HC. . 1997 J. Invest. Dermatol. 109: 163–169.
Hall EJ, Astor M, Bedford J, Borek C, Curtis SB, Fry M, Geard C, Hei T, Mitchell J and Oleinick N, et al. 1998 Am J. Clin. Oncol. 11: 220–252.
Henseleit U, Rosenbach T and Kolde G. . 1996 Arch. Dermatol. Res. 288: 676–683.
Ho SP, Britton DHO, Sone BA, Behrens DL, Leffet LM, Hobbs FW, Miller JA and Trainor GL. . 1996 Nucl. Acids Res. 24: 1901–1907.
Hockenbery DM, Zutter M, Hickey W, Hahm M and Korsmeyer SJ. . 1991 Proc. Natl. Acad. USA 88: 6961–6965.
Kamb A. . 1994 Nature 372: 730–731.
Kondo S, Shinomura Y, Kanayama S, Higashimoto Y, Miyagawa JI, Minami T, Kiyohara T, Zushi S, Kitamura S, Isozaki K and Matsuzawa Y. . 1996 Int. J. Cancer 68: 727–730.
Kirsh EJ, Baunoch DA and Stadler WM. . 1998 J. Urol. 159: 1348–1353.
Krajewska M, Moss SF, Krajewski S, Song K, Holt PR and Reed JC. . 1996 Cancer Res. 56: 2422–2427.
Krajewski S, Krajewska M, Ehrmann J, Sikorska M, Lach B, Chatten J and Reed JC. . 1997 Am. J. Pathol. 150: 805–814.
Krajewski S, Krajewska M, Shabaik A, Wang H-G, Ireie S, Fong L and Reed JC. . 1994 Cancer Res. 54: 5501–5507.
Marvel J, Perkins GR, Lopez Rivas A and Collins MK. . 1994 Oncogene 9: 1117–1122.
McDonnell TJ and Korsmeyer SJ. . 1991 Nature 349: 254–256.
Miyashita T and Reed JC. . 1992 Cancer Res. 52: 5407–5411.
Monia BP, Johnston JF, Ecker DJ, Zounes MA, Lima W and Freier SM. . 1992 J. Biol. Chem. 267: 19954–19962.
Motoyama N, Wang F, Roth K, Sawa H, Nakayama K, Hakayama K, Negishi I, Senju S, Zhang Q, Fugii S and Loh DY. . 1995 Science 267: 1506–1510.
Nunez G, London L, Hockenbery D, Alexander M, McKearn JP and Korsmeyer SJ. . 1990 J. Immunol. 144: 3602–3610.
Ohmori T, Podack ER, Nishio K, Takahashi M, Miyahara Y, Takeda Y, Kubota N, Funayama Y, Ogasawara H and Ohira T, et al. 1993 Biochem. Biophys. Res. Commun. 192: 30–36.
Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB and Recant WM. . 1997 Cancer J. Sci. Am. 3: 230–237.
Oltavi ZN and Korsmeyer SJ. . 1994 Cell 79: 189–192.
Parker SL, Tong T, Bolden S and Wingo PA. . 1996 Ca Cancer J. Clin. 46: 5–27.
Pena JC, Fuchs E and Thompson CB. . 1997 Cell Growth & Differ. 8: 619–629.
Pena JC, Rudin CM and Thompson CB. . 1998 Cancer Res. 58: 2111–2116.
Pollman MJ, Hall JL, Mann MJ, Zhang L and Gibbons GH. . 1998 Nature Med. 4: 222–227.
Ramaswamy NT, Ronai Z and Pelling JC. . 1998 Oncogene 16: 1501–1505.
Reed JC. . 1997 J. Natl. Cancer Inst. 89: 988–990.
Rodeck U, Jost M, DuHadaway J, Kari C, Jensen PJ, Risse B and Ewert DL. . 1997 Proc. Natl. Acad. Sci. USA 94: 5067–5072.
Rodriguez-Villanueva J, Greenhalgh D, Wang X-J, Bundman D, Cho S, Delehedde M, Roop D and McDonnell TJ. . 1998 Oncogene 16: 853–863.
Saini KS and Walker NI. . 1998 Mol. Cell. Biochem. 178: 9–25.
Sanchez Y and Elledge SJ. . 1995 BioEssays 17: 545–548.
Schmitt E, Cimoli G, Steyaert A and Bertrand R. . 1998 Exp. Cell Res. 240: 107–121.
Schwandner R, Wiegmann K, Bernardo K, Kreder D and Kronke M. . 1998 J. Biol. Chem. 273: 5916–5922.
Sentman CL, Shutter JR, Hockenbery D, Kanagawa O and Korsmeyer SJ. . 1991 Cell 67: 879–888.
Sermadiras S, Dumas M, Joly-Berville R, Bonte F, Meybeck A and Ratinaud MH. . 1997 Bri. J. Dermatol. 137: 883–889.
Sierakowska H, Sambade MJ, Agrawal S and Kole R. . 1996 Natl. Acad. Sci. USA 93: 12840–12844.
Stoll SW, Benedict M, Mitra R, Hiniker A, Elder JT and Nunez G. . 1998 Oncogene 16: 1493–1499.
Strasser A, Harris AW and Cory S. . 1991 Cell 67: 889–899.
Tron VA, Trotter MJ, Tang L, Krajewska M, Reed JC, Ho VC and Li G. . 1998 Am. J. Pathol. 153: 579–585.
Vaux DL, Cory S and Adams JM. . 1988 Nature 335: 440–442.
Veis DJ, Sorenson CM, Shutter JR and Korsmeyer SJ. . 1993 Cell 75: 229–240.
Walton MI, Whysong D, O'Connor PM, Hockenbery D, Korsmeyer SJ and Kohn KW. . 1993 Cancer Res. 53: 1853–1861.
Webb A, Cunningham D, Cotter F, Clarke PA, di Stefano F, Ross P, Corbo M and Dziewanowska Z. . 1997 Lancet 349: 1137–1141.
Wrone-Smith T, Johnson T, Nelson B, Boise LH, Thompson CB, Nunez G and Nickoloff BJ. . 1995 Am J. Pathol. 146: 1079–1088.
Yang E and Korsmeyer SJ. . 1996 Blood 88: 386–401.
Acknowledgements
We thank Elizabeth J Ackermann, C Frank Bennett and Thomas P Condon for critical reading of this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, J., Zhang, Q., Monia, B. et al. Inhibition of Bcl-xL expression sensitizes normal human keratinocytes and epithelial cells to apoptotic stimuli. Oncogene 18, 4495–4504 (1999). https://doi.org/10.1038/sj.onc.1202836
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202836
Keywords
This article is cited by
-
ΔNp63α Promotes Apoptosis of Human Epidermal Keratinocytes
Journal of Investigative Dermatology (2007)
-
Distinct promoters mediate constitutive and inducible Bcl-XL expression in malignant lymphocytes
Oncogene (2007)
-
Modulation of 5' splice site selection using tailed oligonucleotides carrying splicing signals
BMC Biotechnology (2006)
-
Bcl-xL antisense oligonucleotides radiosensitise colon cancer cells
British Journal of Cancer (2003)
-
Antisense oligonucleotide-based therapeutics for cancer
Oncogene (2003)