Abstract
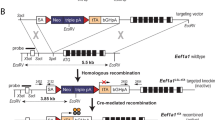
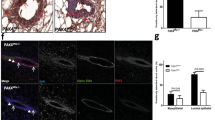
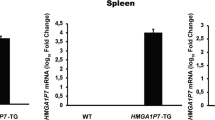
Cdc25 A and B are dual-specificity phosphatases which have been implicated in neoplastic transformation. Although Cdc25A and Cdc25B have been found to be over-expressed in many cancer cell lines and primary tumors, the physiological roles of Cdc25A and B in vivo are largely undefined. To investigate the roles of these proteins in the oncogenic transformation of the mammary gland we used the mouse mammary tumor virus (MMTV) promoter to target over-expression of the Cdc25B transgene in the mammary glands of transgenic mouse lines. Here we report that the over-expression of Cdc25B enhances the proliferation of mammary epithelial cells resulting in the formation of precocious alveolar hyperplasia. At the molecular level, marked increases in cyclin D1 protein have been found in transgenic mammary epithelial cells. The accelerated growth rate of the mammary epithelial cells could also be attributed to the increased levels of cyclin E/cdk2 activity. In addition, a pronounced decrease in apoptosis was also observed during the involution of mammary gland. The reduction of apoptosis during involution correlated well with the reduced expression of c-myc and p53, both of which have been implicated in apoptosis. Taken together, our results clearly indicate that the deregulated expression of Cdc25B generates mammary gland hyperplasia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Arends MJ, McGregor AH, Toft NJ, Brown EJ and Wyllie AH. . 1993 Br. J. Cancer 68: 1127–1133.
Barnes DM. . 1997 J. Pathol. 181: 267–269.
Booher RN, Deshaies RJ and Kirschner MW. . 1993 EMBO J. 12: 3417–3426.
Bortner DM and Rosenberg MP. . 1995 Cell Growth Differ. 6: 1579–1589.
Bortner DM and Rosenberg MP. . 1997 Mol. Cell. Biol. 17: 453–459.
Cardiff RD. . 1996 J. Mammary Gland Biol. Neoplasia 1: 61–73.
Conklin DS, Galaktionov K and Beach D. . 1995 Proc. Natl Acad. Sci. USA 92: 7892–7896.
Draetta G. . 1994 Curr. Opin. Cell Biol. 6: 842–846.
Draetta G and Eckstein J. . 1997 Biochim. Biophys. Acta 1332: M53–M63.
Elledge SJ. . 1996 Science 274: 1664–1672.
Galaktionov K and Beach D. . 1991 Cell 67: 1181–1194.
Galaktionov K, Chen X and Beach D. . 1996 Nature 382: 511–517.
Galaktionov K, Jessus C and Beach D. . 1995a Genes Dev. 9: 1046–1058.
Galakionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M and Beach D. . 1995b Science 269: 1575–1577.
Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S and Boiocchi M. . 1997 Cancer Res. 57: 2366–2368.
Gavrieli Y, Sherman Y and Ben-Sasson SA. . 1992 J. Cell. Biol. 119: 493–501.
Grana X and Reddy EP. . 1995 Oncogene 11: 211–219.
Gray-Bablin J, Zalvide J, Fox MP, Knickerbocker CJ, DeCaprio JA and Keyomarsi K. . 1996 Proc. Natl Acad. Sci. USA 93: 15215–15220.
Gunzburg WH and Salmons B. . 1992 Biochem. J. 283: 625–632.
Harper JW and Elledge SJ. . 1996 Curr. Opin. Genetics Dev. 6: 56–64.
Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L and Swindell E, Fox MP and Wei N. . 1995 Mol. Biol. Cell. 6: 387–400.
Hernandez S, Hernandez L, Bea S, Cazorla M, Fernandez PL, Nadal A, Muntane J, Mallofre C, Montserrat E, Cardesa A and Campo E. . 1998 Cancer Res. 58: 1762–1767.
Hirai H, Roussel MF, Kato JY, Ashmun RA and Sherr CJ. . 1995 Mol. Cell. Biol. 15: 2672–2681.
Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, Reed JC and Rosen JM. . 1996 Development 122: 4013–4022.
Hundley JE, Koester SK, Troyer DA, Hilsenbeck SG, Barrington RE and Windle JJ. . 1997 Cancer Res. 57: 600–603.
Hunter T and Pines J. . 1994 Cell 79: 573–582.
Iavarone A and Massague J. . 1997 Nature 387: 417–422.
Igarashi M, Nagata A, Jinno S, Suto K and Okayama H. . 1991 Nature 353: 80–83.
Jerry DJ, Kuperwasser C, Downing SR, Pinkas J, He C, Dickinson E, Marconi S and Naber SP. . 1998 Oncogene 17: 2305–2312.
Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H and Okayama H. . 1994 EMBO J. 13: 1549–1556.
Keyomarsi K and Pardee AB. . 1993 Proc. Natl. Acad. Sci. USA 90: 1112–1116.
Kudo Y, Yasui W, Ue T, Yamamoto S, Yokozaki H, Nikai H and Tahara E. . 1997 Jpn J. Cancer Res. 88: 947–952.
Li B, Greenberg N, Stephens LC, Meyn R, Medina D and Rosen JM. . 1994 Cell Growth Differ. 5: 711–721.
Matsui Y, Halter SA, Holt JT, Hogan BL and Coffey RJ. . 1990 Cell 61: 1147–1155.
Medina D. . 1996 J. Mammary Gland Biol. Neoplasia 1: 5–18.
Millar JB, McGowan CH, Lenaers G, Jones R and Russell P. . 1991 EMBO J. 10: 4301–4309.
Moreno S and Nurse P. . 1991 Nature 351: 194.
Morgan DO. . 1996 Nature 382: 295–296.
Mueller PR, Coleman TR, Kumagai A and Dunphy WG. . 1995 Science 270: 86–90.
Pattengale PK, Stewart TA, Leder A, Sinn E, Muller W, Tepler I, Schmidt E and Leder P. . 1989 Am. J. Pathol. 135: 39–61.
Pines J. . 1993 Trends Biochem. Sci. 18: 195–197.
Robinson GW, McKnight RA, Smith GH and Hennighausen L. . 1995 Development 121: 2079–2090.
Russell P and Nurse P. . 1986 Cell 45: 145–153.
Sadhu K, Reed SI, Richardson H and Russell P. . 1990 Proc. Natl. Acad. Sci. USA 87: 5139–5143.
Saha P, Eichbaum Q, Silberman ED, Mayer BJ and Dutta A. . 1997 Mol. Cell. Biol. 17: 4338–4345.
Serrano M, Hannon GJ and Beach D. . 1993 Nature 366: 704–707.
Sherr CJ. . 1996 Science 274: 1672–1677.
Sherr CJ and Roberts JM. . 1995 Genes Dev. 9: 1149–1163.
Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ and Weinberg RA. . 1995 Cell 82: 621–630.
Sinn E, Muller W, Pattengale P, Tepler I, Wallace R and Leder P. . 1987 Cell 49: 465–475.
Stewart TA, Pattengale PK and Leder P. . 1984 Cell 38: 627–637.
Strange R, Li F, Saurer S, Burkhardt A and Friis RR. . 1992 Development 115: 49–58.
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A and Schmidt EV. . 1994 Nature 369: 669–671.
Wickramasinghe D, Becker S, Ernst MK, Resnick JL, Centanni JM, Tessarollo L, Grabel LB and Donovan PJ. . 1995 Development 121: 2047–2056.
Wu W, Fan YH, Kemp BL, Walsh G and Mao L. . 1998 Cancer Res. 58: 4082–4085.
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R and Beach D. . 1993 Nature 366: 701–704.
Xu X and Burke SP. . 1996 J. Biol. Chem. 271: 5118–5124.
Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ and Roussel MF. . 1998 Genes Dev. 12: 2424–2433.
Acknowledgements
We would like to thank Drs Daniel Medina, Jeffery Gimble, Mong-Hong Lee, Ming-Jer Tsai and Fred A Pereira for their valuable discussions. We are also grateful to Drs David Beach and Brigid Hogan for their generous gift of the human Cdc25B and MMTV-KCR plasmids respectively. Last but not least, we would also like to extend our acknowledgements to Lei Gong, LouAnn Hadsell and John Stockton for their excellent technical support. This work was supported by a grant of DAMD 1794J4400 to SYT.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ma, ZQ., Chua, S., DeMayo, F. et al. Induction of mammary gland hyperplasia in transgenic mice over-expressing human Cdc25B. Oncogene 18, 4564–4576 (1999). https://doi.org/10.1038/sj.onc.1202809
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202809
Keywords
This article is cited by
-
Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies
Molecular and Cellular Biochemistry (2016)
-
Potent CDC25B and PTP1B phosphatase inhibitors: 2′,4′,6′-trihydroxylchalcone derivatives
Medicinal Chemistry Research (2015)
-
LGH00031, a novel ortho-quinonoid inhibitor of cell division cycle 25B, inhibits human cancer cells via ROS generation
Acta Pharmacologica Sinica (2009)
-
Mammary Involution and Breast Cancer Risk: Transgenic Models and Clinical Studies
Journal of Mammary Gland Biology and Neoplasia (2009)
-
Small interfering RNA targeting CDC25B inhibits liver tumor growth in vitro and in vivo
Molecular Cancer (2008)