Abstract
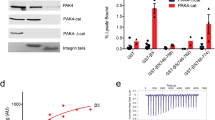
CRKL, an SH2-SH3-SH3 adapter protein, is one of the major tyrosine phosphoproteins detected in primary leukemic neutrophils from patients with CML. CRKL binds directly to BCR/ABL through its N-terminal SH3 domain, suggesting it may be involved in BCR/ABL signal transduction. However, the biological function of CRKL in either normal or leukemic cells is still largely unknown. In this study, we have examined the effects of overexpressing full length or deletion mutants of CRKL in hematopoietic cell lines. Full length, SH2- and SH3(N)-domain deletion mutants of CRKL were transfected into an interleukin-3-dependent hematopoietic cell line, Ba/F3, and 3 – 5 individual sublines which stably overexpressed each transgene were obtained [Ba/F-CRKL, Ba/F-CRKLΔSH2, and Ba/F-CRKL ΔSH3(N)]. The growth properties of these transfected cells in the presence or absence of IL-3 were not different from mock transfected or untransfected Ba/F3 cells. However, Ba/F3 cells overexpressing full length CRKL, but not deletion mutants of CRKL, were found to have an increase in their ability to bind to fibronectin-coated surfaces. Further, expression of full length, but not ΔSH2- or ΔSH3-CRKL deletion mutants, was found to alter cell morphology on fibronectin-coated plates, an effect which was further enhanced by certain kinds of stress stimuli, such as ionizing radiation. Similar results were obtained when CRKL was transiently overexpressed in Ba/F3 cells, and were also obtained in a second IL-3 dependent hematopoietic cell line, 32Dcl3. Adhesion to fibronectin was blocked by anti-β1 integrin monoclonal antibody, but overexpression of CRKL did not affect surface expression of β1 integrins, nor did it spontaneously induce expression of the β1 integrin `activation' epitope recognized by the 9EG7 monoclonal antibody. These data suggest a role for CRKL in signaling pathways which regulate adhesion to fibronectin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Altun-Gultekin ZF, Chandriani S, Bougeret C, Ishizaki T, Narumia S, deGraaf P, Van Bergen en Henegouwen P, Hanafusa H, Wagner JA and Birge RB. . 1998 Mol. Cell. Biol. 18: 3044–3058.
Andoniou CE, Thien CB and Langdon WY. . 1996 Oncogene 12: 1981–1989.
Barber DL, Mason JM, Fukazawa T, Reedquist KA, Druker BJ, Band H and D'Andrea AD. . 1997 Blood 89: 3166–3174.
Bazzoni G, Carlesso N, Griffin JD and Hemler ME. . 1996 J. Clin. Invest. 98: 521–528.
Bellis SL, Perrotta JA, Curtis MS and Turner CE. . 1997 Biochem. J. 15: 375–381.
Birge RB, Fajardo JE, Mayer BJ and Hanafusa H. . 1992 J. Biol. Chem. 267: 10588–10595.
Birge RB, Fajardo JE, Reichman C, Shoelson SE, Songyang Z, Cantley LC and Hanafusa H. . 1993 Mol. Cell. Biol. 13: 4648–4656.
Chin H, Saito T, Arai A, Yamamoto K, Kamiyama R, Miyasaka N and Miura O. . 1997 Biochem. Biophys. Res. Commun. 239: 412–417.
de Jong R, ten Hoeve J, Heisterkamp N and Groffen J. . 1995 J. Biol. Chem. 270: 21468–21471.
de Jong R, ten Hoeve J, Heisterkamp N and Groffen J. . 1997 Oncogene 14: 507–513.
Feller SM, Knudsen B and Hanafusa H. . 1994 EMBO J. 13: 2341–2351.
Feller SM, Knudsen B and Hanafusa H. . 1995 Oncogene 10: 1465–1473.
Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, Kurata T and Matsuda M. . 1996 Mol. Cell. Biol. 16: 1770–1776.
Ichiba T, Kuraishi Y, Sakai O, Nagata S, Groffen J, Kurata T, Hattori S and Matsuda M. . 1997 J. Biol. Chem. 272: 22215–22220.
Jacobson K, Kravitz J, Kincade PW and Osmond DG. . 1996 Blood 87: 73–82.
Kanner SB, Reynolds AB, Wang HC, Vines RR and Parsons JT. . 1991 EMBO J. 10: 1689–1698.
Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y and Noda M. . 1989 Cell 56: 77–84.
Klemke RL, Leng J, Molander R, Brooks PC, Vuori K and Cheresh DA. . 1998 J. Cell Biol. 140: 961–972.
Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B and Vestweber D. . 1993 Proc. Natl. Acad. Sci. USA 90: 9051–9055.
Lewis JM, Baskaran R, Taagepera S, Schwartz MA and Wang JY. . 1996 Proc. Natl. Acad. Sci. USA 93: 15174–15179.
Lo SH, Weisberg E and Chen LB. . 1994 Bioessays 16: 817–823.
Manie SN, Beck AR, Astier A, Law SF, Canty T, Hirai H, Druker BJ, Avraham H, Haghayeghi N, Sattler M, Salgia R, Griffin JD, Golemis EA and Freedman AS. . 1997 J. Biol. Chem. 272: 4230–4236.
Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T and Shibuya M. . 1992 Mol. Cell. Biol. 12: 3482–3489.
Matulonis U, Salgia R, Okuda K, Druker B and Griffin JD. . 1993 Exp. Hematol. 21: 1460–1466.
Mayer BJ, Hamaguchi M and Hanafusa H. . 1988 Nature 332: 272–275.
Mayer BJ, Hirai H and Sakai R. . 1995 Curr. Biol. 5: 296–305.
Mizushima S and Nagata S. . 1990 Nucl. Acids Res. 18: 5322.
Nichols GL, Raines MA, Vera JC, Lacomis L, Tempst P and Golde DW. . 1994 Blood 84: 2912–2918.
Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C and Yazaki Y, et al. 1995 J. Biol. Chem. 270: 15398–15402.
Oda T, Heaney C, Hagopian JR, Okuda K, Griffin JD and Druker BJ. . 1994 J. Biol. Chem. 269: 22925–22928.
Onoda JM, Piechocki MP and Honn KV. . 1992 Radiation Res. 130: 281–288.
Park H-O, Bi E, Pringle JR and Herskowitz I. . 1997 Proc. Natl. Acad. Sci. USA 94: 4463–4468.
Polte TR and Hanks SK. . 1995 Proc. Natl. Acad. Sci. USA 92: 10678–10682.
Rebstein PJ, Cardelli J, Weeks G and Spiegelman GB. . 1997 Exp. Cell Res. 231: 276–283.
Ren R, Zheng-Sheng Y and Baltimore D. . 1994 Genes Dev. 8: 783–795.
Rosen MK, Yamazaki T, Gish GD, Kay CM, Pawson T and Kay LE. . 1995 Nature 374: 477–479.
Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y and Hirai H. . 1994 EMBO J. 13: 3748–3756.
Salgia R, Li J-L, Lo SH, Brunkhorst B, Kansas GS, Sobhany ES, Sun Y, Pisick E, Hallek M, Ernst T, Tantravahi R, Chen LB and Griffin JD. . 1995a J. Biol. Chem. 270: 5039–5047.
Salgia R, Pisick E, Sattler M, Li JL, Uemura N, Wong WK, Burky SA, Hirai H, Chen LB and Griffin JD. . 1996a J. Biol. Chem. 271: 25198–25203.
Salgia R, Sattler M, Pisick E, Li JL and Griffin JD. . 1996b Exp. Hematol. 24: 310–313.
Salgia R, Uemura N, Okuda K, Li JL, Pisick E, Sattler M, de Jong R, Druker B, Heisterkamp N, Chen LB and Griffin JD. . 1995b J. Biol. Chem. 270: 29145–29150.
Sattler M, Salgia R, Okuda K, Uemura N, Durstin MA, Pisick E, Xu G, Li JL, Prasad KV and Griffin JD. . 1996 Oncogene 12: 839–846.
Sattler M, Salgia R, Shrikhande G, Verma S, Pisick E, Prasad KV and Griffin JD. . 1997a J. Biol. Chem. 272: 10248–10253.
Sattler M, Salgia R, Shrikhande G, Verma S, Uemura N, Law SF, Golemis EA and Griffin JD. . 1997b J. Biol. Chem. 272: 14320–14326.
Sawasdikosol S, Ravichandran KS, Lee KK, Chang JH and Burakoff SJ. . 1995 J. Biol. Chem. 270: 2893–2896.
Schumacher C, Knudsen BS, Ohuchi T, Di Fiore PP, Glassman RH and Hanafusa H. . 1995 J. Biol. Chem. 270: 15341–15347.
Senechal K, Halpern J and Sawyers CL. . 1996 J. Biol. Chem. 271: 23255–23261.
Senechal K, Heaney C, Druker B and Sawyers CL. . 1998 Mol. Cell. Biol. 18: 5082–5090.
Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, Neel BG, Birge RB, Fajardo JE, Chou MM, Hanafusa H, Schaffhausen B and Cantley LC. . 1993 Cell 72: 767–778.
Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, Matuoka K, Takenawa T, Kurata T and Nagashima K, et al. 1994 Proc. Natl. Acad. Sci. USA 91: 3443–3447.
ten Hoeve J, Arlinghaus RB, Guo JQ, Heisterkamp N and Groffen J. . 1994a Blood 84: 1731–1736.
ten Hoeve J, Kaartinen V, Fioretos T, Haataja L, Voncken JW, Heisterkamp N and Groffen J. . 1994b Cancer Res. 54: 2563–2567.
ten Hoeve J, Morris C, Heisterkamp N and Groffen J. . 1993 Oncogene 8: 2469–2474.
Uemura N, Salgia R, Li JL, Pisick E, Sattler M and Griffin JD. . 1997 Leukemia 11: 376–385.
White GC, Crawford N and Fischer TH. . 1993 Adv. Exp. Med. Biol. 344: 187–194.
Acknowledgements
This work was supported by NIH grants CA36167 and DK560654 (JDG).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uemura, N., Salgia, R., Ewaniuk, D. et al. Involvement of the adapter protein CRKL in integrin-mediated adhesion. Oncogene 18, 3343–3353 (1999). https://doi.org/10.1038/sj.onc.1202689
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202689
Keywords
This article is cited by
-
C3G forms complexes with Bcr-Abl and p38α MAPK at the focal adhesions in chronic myeloid leukemia cells: implication in the regulation of leukemic cell adhesion
Cell Communication and Signaling (2013)
-
Molecular Biology of Chronic Myeloid Leukemia
International Journal of Hematology (2001)