Abstract
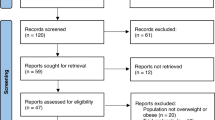
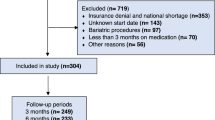
This study aims to determine the efficacy and tolerability of sibutramine hydrochloride in overweight and obese patients with cardiovascular risk factors. This was a 12-week, open-label, observational trial carried out in primary care settings. Patients' data were obtained from questionnaires received from 153 physicians. A total of 2225 overweight and obese (BMI⩾27 kg/m2) patients received sibutramine in single daily doses of 10 and/or 15 mg. The study population included patients in general good health and with controlled hypertension (41.2%), type II diabetes mellitus (15.6%), hyperlipidaemia (45.5%), and who were chronic tobacco users (smokers) (37.0%). The main outcome measures were changes in body weight, blood pressure and heart rate, and evaluation of reported adverse events. Reduction of body weight of at least >5% from baseline to week 12 was achieved in 2030 (91%) patients and >10% was achieved in 987 (44%) patients. Baseline differences in the percentages of male and female patients, presence or absence of hyperlipidaemia or smoking status did not appear to affect the rate of weight change. Weight loss was less in patients with type II diabetes mellitus and/or controlled systolic hypertension at baseline compared to those patients without these conditions. Mean systolic and diastolic blood pressure and heart rate decreased from baseline to week 12. Overall, sibutramine was well tolerated. In conclusions, treatment with sibutramine resulted in clinically significant weight loss during short-term therapy in obese adults with a range of cardiovascular risk factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure: the trials of hypertension prevention, phase II. Arch Intern Med 1997; 157: 657–667.
Sharma AM, Engell S, Pischon T . New developments in mechanisms of obesity-induced hypertension: role of adipose tissue. Curr Hypertens Rep 2001; 3: 152–156.
Howard BV, Ruotolo G, Robbins DC . Obesity and dyslipidemia. Endocrinol Metab Clin North Am 2003; 32: 855–867.
Messerli FH, Ketelhut R . Left ventricular hypertrophy: a pressure-independent cardiovascular risk factor. J Cardiovasc Pharmacol 1993; 22(Suppl 1): S7–S13.
Dattilo AM, Kris-Etherton PM . Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr 1992; 56: 320–328.
Zannad F et al. Effects of sibutramine on ventricular dimensions and heart valves in obese patients during weight reduction. Am Heart J 2002; 144: 508–515.
Scholtze J . Treating obesity with sibutramine under practical conditions. Positive effects on the metabolic parameters and blood pressure (English translation). Dtsh Med Wochenschr 2002; 127: 606–610.
Mertens IL, Van Gaal LF . Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res 2000; 8: 270–278.
Tuck ML et al. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 1981; 304: 930–933.
Schotte DE, Stunkard AJ . The effects of weight reduction on blood pressure in 301 obese patients. Arch Intern Med 1990; 150: 1701–1704.
Hansen DL et al. The effect of sibutramine on energy expenditure and appetite during chronic treatment without dietary restriction. Int J Obes Relat Metab Disord 1999; 23: 1016–1024.
Walsh KM, Leen E, Lean MEJ . The effect of sibutramine on resting energy expenditure and adrenaline-induced thermogenesis in obese females. Int J Obes Relat Metab Disord 1999; 23: 1009–1015.
Bray GA et al. Sibutramine produces dose-related weight loss. Obes Res 1999; 7: 189–198.
Package insert. Reductil/Meridia (sibutramine). Abbott Laboratories, 2004.
MacMahon FG et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension: a 1-year, double-blind, placebo-controlled, multicenter trial. Arch Intern Med 2000; 160: 2185–2191.
McMahon FG et al. Sibutramine is safe and effective for weight loss in obese patients whose hypertension is well controlled with angiotensin-converting enzyme inhibitors. J Hum Hypertens 2002; 16: 5–11.
Sramek JJ et al. Efficacy and safety of sibutramine for weight loss in obese patients with hypertension well controlled by β-adrenergic blocking agents: a placebo-controlled, double-blind, randomised trial. J Hum Hypertens 2002; 16: 13–19.
World Health Organization (homepage on the Internet), Geneva, Switzerland (updated 2003 March 9; cited 2004 December 13). Available from http://www.who.int/nut/obs.htm.
World Health Organization. World Health Report 2002: Reducing Risks, Promoting Healthy Life. Executive Summary. WHO Publications, Geneva, 2002, p 1.
World Health Organization. Obesity: Preventing and Managing the Global Epidemic, Report of World Health Organization Consultation on Obesity, Executive Summary. Technical Report Series. WHO Publications, Geneva, 2000, p 1.
World Health Organization. World Health Report 2002: Reducing Risks, Promoting Healthy Life. WHO Publications, Geneva, 2002, p 8.
World Health Organization Report. Obesity: Preventing and Managing the Global Epidemic. Technical Report Series. WHO Publications, Geneva, 2000, p 25.
International Obesity Task Force [homepage on the Internet]. London, UK. Link: About Obesity. Page: The Developing World's New Burden: Obesity [cited 2004 Dec 13]. Available from: www.iotf.org.
National Institutes of Health; National Heart, Lung, and Blood Institute; North American Association for the Study of Obesity. The Practical Guide 2000, p 5.
Wirth A, Krause J . Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001; 286: 1331–1339.
Tuomilehto J et al. Prevention of type II diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med 2001; 344: 1343–1350.
Williamson DF . Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40–64 years. Am J Epidemiol 1995; 141: 1128–1141; Erratum, Am J Epidemiol 1995;142:369.
World Health Organization Report. Obesity: Preventing and Managing the Global Epidemic. Technical Report Series. WHO Publications, Geneva, 2000, p 70.
National Task Force on the Prevention and Treatment of Obesity. Long-term pharmacotherapy in the management of obesity. JAMA 1996; 276: 1907–1908.
Astrup A, Chong E . Weight loss produced by sibutramine: a meta-analysis (abstract). Int J Obes Relat Metab Disord 2001; 25(Suppl 2): S104.
James WPT et al. Effect of sibutramine on weight maintenance after weight loss: a randomized trial. Lancet 2000; 356: 2119–2125.
James WPT et al. Effect of sibutramine on weight maintenance after weight loss: a randomized trial (STORM). Lancet 2000; 356: 2119–2125.
Lean ME . Sibutramine — a review of clinical efficacy (review). Int J Obes Relat Metab Disord 1997; 21(Suppl 1): S30–S36; discussion 37–39.
Sharma AM . Sibutramine in overweight/obese hypertensive patients. Int J Obes Relat Metab Disord 2001; 25(Suppl 4): S20–S23.
National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28: 1039.
Smith IG, Goulder MA . Randomized placebo-controlled trial of long-term treatment with sibutramine in mild to moderate obesity. J Fam Pract 2001; 50: 505–512.
Apfelbaum M et al. Long-term maintenance of weight loss after a very-low-calorie diet: a randomized blinded trial of the efficacy and tolerability of sibutramine. Am J Med 1999; 106: 179–184.
Wing RR, Marcus MD, Epstein LH, Salata R . Type II diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care 1987; 10: 563–566.
Finer N et al. Sibutramine is effective for weight loss and diabetic control in obesity with type II diabetes: a randomised, double-blind, placebo-controlled study. Diabetes Obes Metab 2000; 2: 105–112.
Sanchez-Reyes L et al. Use of sibutramine in overweight adult Hispanic patients with type II diabetes mellitus: a 12-month, randomized, double-blind, placebo-controlled clinical trial. Clin Ther 2004; 26: 1427–1435.
McNulty SJ, Ur E, Williams G . A randomized trial of sibutramine in the management of obese type 2 diabetic patients treated with metformin. Diabetes Care 2003; 26: 125–131.
Serrano-Rios M, Melchionda N, Moreno-Carretero E . Role of sibutramine in the treatment of obese type 2 diabetic patients receiving sulphonylurea therapy. Diabet Med 2002; 19: 119–124.
James WPT et al. Effect of sibutramine on weight maintenance after weight loss: a randomized trial (STORM). Lancet 2000; 356: 2119–2125.
Lean ME . Sibutramine — a review of clinical efficacy (review). Int J Obes Relat Metab Disord 1997; 21(Supp 1): S30–S36; discussion 37–39.
Sharma AM . Sibutramine in overweight/obese hypertensive patients. Int J Obes Relat Metab Disord 2001; 25(Suppl 4): S20–S23.
Arterburn DE, Crane PK, Veenstra DL . The efficacy and safety of sibutramine for weight loss: a systematic review. Arch Intern Med 2004; 164(Suppl 9): 994–1003.
Narkiewicz K . Sibutramine and its cardiovascular profile. Int J Obes Relat Metab Disord 2002; 26(Suppl 4): S38–S41.
Birkenfeld AL et al. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation 2002; 106: 2459–2465.
Godoy-Matos A . Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab 2005; 90: 1460–1465.
Jordan J et al. Influence of sibutramine on blood pressure: evidence from placebo-controlled trials. Int J Obes Relat Metab Disord (Advance E-pub) 2005; doi 10.1038/sj.ijo.0802887.
Hazenberg BP . Randomized, double-blind, placebo-controlled, multicenter study of sibutramine in obese hypertensive patients. Cardiology 2000; 94: 152–158.
Acknowledgements
The study was supported by a Grant from Abbott Laboratories.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Gaciong, Z., Placha, G. & for Polish Sibutramine Study Group. Efficacy and safety of sibutramine in 2225 subjects with cardiovascular risk factors: short-term, open-label, observational study. J Hum Hypertens 19, 737–743 (2005). https://doi.org/10.1038/sj.jhh.1001877
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jhh.1001877
Keywords
This article is cited by
-
Safety Assessment of an Anti-Obesity Drug (Sibutramine)
Drug Safety (2012)
-
Association of CYP2B6, CYP3A5, and CYP2C19 Genetic Polymorphisms With Sibutramine Pharmacokinetics in Healthy Korean Subjects
Clinical Pharmacology & Therapeutics (2009)