Abstract
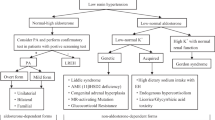
Recently published studies from different parts of the world report significantly higher prevalence of primary hyperaldosteronism (PH) in hypertensives (ranging from 5 to 25%) than the previously accepted figures. There have been no data so far about the prevalence of PH in Central Europe. Therefore, we have undertaken this study to evaluate the prevalence of PH in patients with moderate to severe hypertension referred to a hypertension unit in the Czech Republic, together with the determination of the percentage of different subtypes of PH including familial forms. In addition to that, we have evaluated the prevalence of other types of secondary forms of hypertension.
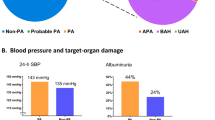
A total of 402 consecutive patients (230 females and 172 males) with hypertension, referred to our hypertension unit, were studied. Positive aldosterone/renin ratio (ARR, (ng/100 ml)/(ng/ml/h)) ⩾50 as a more strict marker of PH was found in 87 patients (21.6%), 30% of them were normokalaemic. The diagnosis of PH was later confirmed in 77 cases (89%); the total prevalence of PH was thus 19%. PH consisted of the following forms: idiopathic hyperaldosteronism 42%, unilateral aldosterone-producing adenoma 36%, unilateral hyperplasia 7%, nonclassifiable PH (refused operation/adrenal venous sampling) 13%, familial hyperaldosteronism type 1.2%. The prevalence of other types of secondary hypertension was as follows: pheochromocytoma 5%, renovascular 4.5%, hypercortisolism 2%, renal 0.75%.
In conclusion, we have noted that PH in the Central Europe region (Czech Republic) is the most frequent form of endocrine hypertension with a considerably high prevalence in moderate to severe hypertension. Application of more strict criteria raises the probability of correct diagnosis of PH including the early normokalaemic stages of PH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Conn JW . Primary aldosteronism, a new clinical syndrome. J Lab Clin Med 1955; 45: 16–17.
Conn JW . The evolution of primary aldosteronism 1954–1967. Harvey Lect 1968; 62: 257–291.
Hiramatsu et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Arch Intern Med 1981; 141: 1589–1593.
Fardella C et al. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile and molecular biology. J Clin Endocrinol Metab 2000; 85: 1863–1867.
Loh K et al. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab 2000; 85: 2854–2859.
Lim PO et al. High prevalence of primary aldosteronism in the Tayside hypertension clinic population. J Hum Hypertens 2000; 14: 311–315.
Stowasser M . Primary aldosteronism: rare bird or common cause of secondary hypertension? Curr Hypertens Rep 2001; 3: 230–239.
Gordon RD, Stowasser M . Primary aldosteronism: are we diagnosing and operating on too few patients? World J Surg 2001; 25: 941–947.
Kaplan NM . Cautious over the current epidemic of primary aldosteronism. Lancet. London 2001; 357: 953–954.
Ganguly A . Primary aldosteronism. N Engl J Med 1998; 339: 1828–1834.
Peters J et al. Molekular-biologie. Klinik und Therapie steroidbedingter Hypertonien. In: Ganten D, Ruckpaul K (eds). Handbuch der Molekularen Medizin. Springer-Verlag: Berlin, 1998, pp 413–452.
Seeman T, Widimsky J, Hampf M, Bernhardt R . Abolished nocturnal blood pressure fall in a boy with glucocorticoid-remediable aldosteronism. J Hum Hypertens. 1999; 13: 823–828.
Magill SB et al. Comparison of adrenal venous sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab. 2001; 86: 1067–1071.
Gordon RD, Stowasser M, Klemm SA, Tunny TJ . Primary aldosteronism and other forms of mineralocorticoid hypertension. In: Swales JD (ed). Textbook of Hypertension. Blackwell Scientific Publications: London, 1994, pp 865–892.
Gordon RD et al. Evidence that primary aldosteronism may not be uncommon: 12% incidence among anti-hypertensive drug trail volunteers. Clin Exp Pharmacol Physiol 1993; 20: 296–298.
Acknowledgements
This study was supported by Project No J 13198: 11110000-2 of the Ministry of Education, Youth and Sports of the Czech Republic
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Štrauch, B., Zelinka, T., Hampf, M. et al. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens 17, 349–352 (2003). https://doi.org/10.1038/sj.jhh.1001554
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jhh.1001554
Keywords
This article is cited by
-
Somatic SLC30A1 mutations altering zinc transporter ZnT1 cause aldosterone-producing adenomas and primary aldosteronism
Nature Genetics (2023)
-
Postoperative adrenal insufficiency in Conn’s syndrome—does it occur frequently?
Journal of Human Hypertension (2022)
-
Determination of adrenal hypersecretion in primary Aldosteronism without aldosterone-production adenomas
BMC Endocrine Disorders (2021)
-
Eplerenone levels in maternal serum, cord blood, and breast milk during pregnancy and lactation
Hypertension Research (2021)
-
Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism
Hypertension Research (2021)