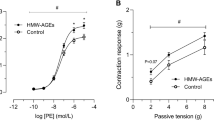
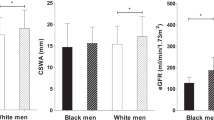
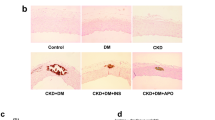
Abstract
Enhanced oxidative stress is involved in the progression of renal disease. Since angiotensin converting enzyme inhibitors (ACEI) have been shown to improve the antioxidative defence, we investigated, in patients with nondiabetic nephropathy, the short-term effect of the ACEI ramipril on parameters of oxidative stress, such as advanced glycation end products (AGEs), advanced oxidation protein products (AOPPs), homocysteine (Hcy), and lipid peroxidation products. Ramipril (2.5–5.0 mg/day) was administered to 12 newly diagnosed patients for 2 months and data compared with a patient group under conventional therapy (diuretic/β-blockers) and with age- and sex-matched healthy subjects (CTRL). Patients had mild to moderate renal insufficiency and showed, in the plasma, higher fluorescent AGE and carboxymethyllysine (CML) levels, as well as elevated concentrations of AOPPs, lipofuscin and Hcy when compared with CTRL. Basal data of the patients on conventional therapy did not differ significantly from the ramipril group, except for higher Hcy levels in the latter. Administration of ramipril resulted in a drop in blood pressure and proteinuria, while creatinine clearance remained the same. The fluorescent AGEs exhibited a mild but significant decline, yet CML concentration was unchanged. The AOPP and malondialdehyde concentrations decreased, while a small rise in neopterin levels was evident after treatment. The mentioned parameters were not affected significantly in the conventionally treated patients. Evidence that ramipril administration results in a mild decline of fluorescent AGEs is herein presented for the first time. The underlying mechanism may be decreased oxidative stress, as indicated by a decline in AOPPs and malondialdehyde.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siems W et al. Oxidative stress in chronic renal failure as a cardiovascular risk factor. Clin Nephrol 2002; 58 (Suppl 1): S12–S19.
Stenvinkel P et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 1999; 55: 1899–1911.
Ritz E, Deppisch R, Nawroth P . Toxicity or uraemia: does it come of AGE? Nephrol Dial Transplant 1994; 9: 1–2
Degenhardt TP et al. The serum concentration of the advanced glycation end product Nɛ-(carboxymethyl)lysine is increased in uremia. Kidne Int 1997; 52: 1064–1067.
Miyata T et al. Accumulation of albumin-linked and free form pentosidine in circulation of uremic patients with end stage renal failure: renal implications in pathophysiology of pentosidine. J Am Soc Nephrol 1996; 7: 1198–1206.
Šebeková K et al. Plasma levels of advanced glycation end products in children with renal disease. Ped Nephrol 2001; 16: 1105–1112.
Miyata T et al. Alterations in non-enzymatic glycation in uremia: origin and significance of ‘carbonyl stress’ in long term uremic complications. Kidney Int 1955; 55: 389–399.
Miyata T et al. Implication of an increased oxidative stress in the formation of advanced glycation end products in patients with end-stage renal failure. Kidney Int 1997; 51: 1170–1181.
Bucala R . Lipid and lipoprotein modification by AGEs: role in atherosclerosis. Exp Physiol 1997; 82: 327–337.
Thornalley PJ . Cell activation by glycated proteins, AGE receptors, receptor recognition factors and functional classification of AGE. Cell Mol Biol 1988; 44: 1013–1023.
Perna AF, Castaldo P, Ingrosso D, De Santo NG . Homocysteine, a new cardiovascular risk factor, is also a powerful uremic toxin. J Nephrol 1999; 12: 230–240.
Welch GN, Loscalzo JL . Homocysteine and atherothrombosis. N Engl J Med 1998; 338: 1024–1050.
Witko-Sarsat V et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996; 49: 1304–1313.
Chopra M et al. Antioxidants effects of angiotensin-converting enzyme (ACE) inhibitors: free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl-and non-sulfhydryl-containing ACE inhibitors. J Cardiovasc Pharmacol 1992; 19: 330–340.
Miyata T et al. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol 2002; 13: 2478–2487.
De Cavanagh EM, Inserra F, Ferder L, Fraga CG . Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 2000; 278: R572–R577.
Kedziora-Kornatowska KZ, Luciak M, Paszkowski J . Lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney: effect of treatment with angiotensin convertase inhibitors. IUBMB Life 2000; 49: 303–307.
Tsutsui H et al. Effects of ACEI inhibition on left ventricular failure and oxidative stress in Dahl salt-sensitive rats. J Cardiovasc Pharmacol 2001; 37: 725–733.
Tesar V et al. Influence of losartan and enalapril on urinary excretion of 8-isoprostane in experimental nephrotic syndrome. Med Sci Monit 2002; 8: BR69–BR74.
Münch G et al. Determination of advanced glycation end products in serum by fluorescene spectroscopy and competitive ELISA. Eur J Clin Chem 1997; 35: 669–677.
Gerdemann A et al. Low molecular but not high molecular AGEs are removed by high flux hemodialysis. Clin Nephrol 2001; 54: 276–283.
Mellinghoff AC et al. Formation of plasma advanced glycosylation end-products (AGEs) has no influence on plasma viscosity. Diabet Med 1997; 14: 832–836.
Tschuchida M et al. Fluorescent substances in mouse and human sera as a parameter of in vivo lipoperoxidation. Biochim Biophys Acta 1985; 843: 214–220.
Vester B, Rasmussen K . High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem 1991; 29: 549–554.
Descamps-Latscha B, Jungers P, Witko-Sarsat V . Immune system dysregulation in uremia: role of oxidative stress. Blood Purif 2002; 20: 481–484.
Brownlee M . Advanced protein glycosylation in diabetics and aging. Annu Rev Med 1995; 46: 223–234.
Horiuchi S et al. Advanced glycosylation end products and their recognition by macrophage derived cells. Diabetes 1996; S3: S73–S76.
Gugliucci A, Bendayan M . Renal fate of circulating advanced glycation end products (AGEs): evidence for absorbtion and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia 1996; 39: 149–160.
SŠ ebeková K, KupcŠová V, Schinzel R, Heidland A . Markedly elevated levels of plasma advanced glyca-tion end products in patients with liver cirrhosis—amelioration by liver transplantation. J Hepatol 2002; 36: 66–71.
Friedlander M et al. The advanced glycation end product pentosidine and monocyte activation in uremia. Clin Nephrol 1996; 45: 379–382.
Schinzel R, Muench G, Heidland A, Šebeková K . Advanced glycation end products in end-stage renal disease and their removal. Nephron 2001; 87: 295–303.
Forbes JM et al. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes 2002; 51: 3274–3282.
SŠebeková K et al. Advanced glycation end products levels in subtotally nephrectomized rats: beneficial effects of angiotensis II receptor 1 antagonist losartan. Mineral Electrolyte Metab 1999; 25: 380–383.
De Cavanagh EM et al. Higher levels of antioxidant defences in enalapril-treated versus non-enalapril-treated hemodialysis patients. Am J Kidney Dis 1999; 34: 445–455
Floris G, Dezso B, Medgyesi GA, Fust G . Effect of angiotensin II on macrophage functions. Immunology 1983; 48: 529–535.
Hahn AW, Jonas U, Buhler FR, Resink TJ . Activation of human peripheral monocytes by angiotensin II. FEBS Lett 1994; 347: 178–180.
Gullestadt L et al. Effect of high- versus low-dose angiotensin-converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol 1999; 34: 2061–2067.
Acknowledgements
This study was supported by a grant from the Slovak Ministry of Health. Support from the Aventis Pharma, s.r.o., Bratislava, Slovakia and the Verein Bekämzurfung der Hochdruck-und Nierenkrankheiten, Würzburg e.V, Germany is acknowledged. We thank Professor MUDr. Miroslav Mikulecký, DrSc. from Institute of Preventive and Clinical Medicine, Bratislava for his expert assistance in statistical analysis and Mr André Klassen from Wuerzburg for an excellent help in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šebeková, K., Gazdíková, K., Syrová, D. et al. Effects of ramipril in nondiabetic nephropathy: improved parameters of oxidatives stress and potential modulation of advanced glycation end products. J Hum Hypertens 17, 265–270 (2003). https://doi.org/10.1038/sj.jhh.1001541
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jhh.1001541
Keywords
This article is cited by
-
Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies
Nutrition & Metabolism (2018)
-
Die Bedeutung von AGEs und ROS bei Atherosklerose
Herz (2010)