Abstract
Objective:
Low testosterone levels have been shown to be predictive for the development of the metabolic syndrome in men. The aim of this study was to describe effects of testosterone deficiency on metabolic syndrome-related parameters in male rats in order to evaluate the rat as a model for the human metabolic syndrome related to low testosterone levels.
Methods:
Male Sprague–Dawley rats were castrated or sham operated at 16 weeks of age and fed either a standard or a high energy diet. Measured parameters were: food intake, body weight, fat distribution, energy expenditure, physical activity and blood/plasma parameters related to glucose and lipid metabolism.
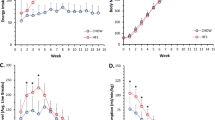
Results:
Castration led to an increase in the amount of subcutaneous fat, but did not result in any changes in the visceral fat. Fasting blood glucose levels were increased and free fatty acids concentration decreased in the castrated rats from 2 weeks after castration and throughout the study, whereas no significant differences between the groups were found in any of the other parameters measured. A high-energy diet did not change the response to castration in male Sprague–Dawley rats.
Conclusion:
Compared to humans rats respond differently to testosterone deficiency. Only few of the features typical for the human metabolic syndrome were observed in castrated male Sprague–Dawley rats. Therefore, we conclude that with the present experimental setup the castrated rat is not an optimal model for studies on the influence of testosterone deficiency on body fat distribution and the development of other central components of the metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alberti KGMM, Zimmet PZ . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional Report of a WHO Consultation. Diabet Med 1998; 15 (7): 539–553.
Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A . Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996; 81: 4358–4365.
Märin P, Holmäng S, Jonsson L, Sjöström L, Kvist H, Holm G et al. The effects of testosterone treatment on body-composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992; 16: 991–997.
Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 1998; 83: 1886–1892.
Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R . Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 1990; 39: 897–901.
Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY . Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 2000; 24: 485–491.
Phillips GB, Jing T, Heymsfield SB . Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism 2003; 52: 784–790.
Phillips GB . Relationship between serum sex hormones and the glucose-insulin-lipid defect in men with obesity. Metabolism 1993; 42: 116–120.
Haffner SM, Mykkänen L, Valdez RA, Katz MS . Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab 1993; 77: 1610–1615.
Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabet Care 2004; 27: 1036–1041.
Muller M, Grobbee DE, den Tonkelaar I, Lamberts SWJ, van der Schouw YT . Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005; 90: 2618–2623.
Gentry RT, Wade GN . Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol 1976; 90: 18–25.
Krotkiewski M, Kral JG, Karlsson J . Effects of castration and testosterone substitution on body composition and muscle metabolism in rats. Acta Physiol Scand 1980; 109: 233–237.
Woodward CJ . A re-evaluation of the anabolic effect of testosterone in rats: interactions with gonadectomy, adrenalectomy and hypophysectomy. Acta Endocrinol 1993; 128: 473–477.
Holmäng A, Björntorp P . The effects of testosterone on insulin sensitivity in male-rats. Acta Physiol Scand 1992; 146: 505–510.
Haug A, Höstmark AT, Spydevold O, Eilertsen E . Hypercholesterolaemia, hypotriacylglycerolaemia and increased lipoprotein lipase activity following orchidectomy in rats. Acta Endocrinol 1986; 113: 133–139.
Matsuda M, DeFronzo RA . Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabet Care 1999; 22: 1462–1470.
Kral JG, Tisell LE . The effects of castration on body composition, adipose tissue cellularity and lipid and carbohydrate metabolism in adult male rats. Acta Endocrinol 1976; 81: 644–654.
Kakolewski JW, Cox VC, Valenstein ES . Sex differences in body-weight change following gonadectomy of rats. Psychol Rep 1968; 22: 547–554.
Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M . Use of orchiectomy and testosterone replacement to explore meal number- to-meal size relationship in male rats. Am J Physiol – Regul Integr Comp Physiol 1999; 276: R1366–R1373.
Clegg DJ, Benoit SC, Fisher ME, Barrera JG, Seeley RJ, Woods SC . Sex hormones determine fat distribution and sensitivity to adiposity signals. Horm Behav 2003; 44: 42.
Ross R, Léger L, Guardo R, De Guise J, Pike BG . Adipose tissue volume measured by magnetic resonance imaging and computerized tomography in rats. J Appl Physiol (Bethesda, MD) 1991; 70: 2164–2172.
Rössner S, Bo WJ, Hiltbrandt E, Hinson W, Karstaedt N, Santago P et al. Adipose tissue determinations in cadavers – a comparison between cross-sectional planimetry and computed tomography. Int J Obes Relat Metab Disord 1990; 14: 893–902.
van der Kooy Seidell JC . Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993; 17: 187–196.
Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S . A novel technique for the determination of body fat by computed tomography. Int J Obes 1983; 7: 437–445.
Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE . Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 1982; 36: 172–177.
Kvist H, Sjöström L, Tylén U . Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 1986; 10: 53–67.
Märin P, Lönn L, Andersson B, Oden B, Olbe L, Bengtsson BA et al. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 1996; 81: 1018–1022.
Sjögren J, Li M, Björntorp P . Androgen hormone binding to adipose tissue in rats. Biochim Biophys Acta – Gen Sub 1995; 1244: 117–120.
Joyner J, Hutley L, Cameron D . Intrinsic regional differences in androgen receptors and dihydrotestosterone metabolism in human preadipocytes. Hormone and Metabolic Research. Hormon- und Stoffwechselforschung. Horm Metab 2002; 34: 223–228.
Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y . Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol 1998; 274 (6 Part 1): C1645–C1652.
Haffner SM, Valdez RA, Mykkänen L, Stern MP, Katz MS . Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism 1994; 43: 599–603.
Pagotto U, Gambineri A, Pelusi C, Genghini S, Cacciari M, Otto B et al. Testosterone replacement therapy restores normal ghrelin in hypogonadal men. J Clin Endocrinol Metab 2003; 88: 4139–4143.
Gautier JF, Mourier A, Dekerviler E, Tarentola A, Bigard AX, Villette JM et al. Evaluation of abdominal fat distribution in noninsulin- dependent diabetes-mellitus – relationship to insulin- resistance. J Clin Endocrinol Metab 1998; 83: 1306–1311.
Pouliot MC, Després JP, Nadeau A, Moorjani S, Prud'homme D, Lupien PJ et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 1992; 41: 826–834.
Ross R, Fortier L, Hudson R . Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabet Care 1996; 19: 1404–1411.
Xu T, Wang X, Hou S, Zhu J, Zhang X, Huang X . Effect of surgical castration on risk factors for arteriosclerosis of patients with prostate cancer. Chin Med J 2002; 115: 1336–1340.
Legrele C, Sutter BC . Glycemia and insulinemia in castrated male rats, fasting and with glucose overload. Glycémie Et Insulinémie Du Rat Mâle Castré, à Jeun Et Sous Surcharge Glucidique. J Physiol 1972; 65 (Suppl): 257A–258A.
Ramamani A, Aruldhas MM, Govindarajulu P . Differential response of rat skeletal muscle glycogen metabolism to testosterone and estradiol. Canad J Physiol Pharmacol 1999; 77: 300–304.
Richard D, Picard F, Lemieux C, Lalonde J, Samson P, Deshaies Y . The effects of topiramate and sex hormones on energy balance of male and female rats. Int J Obes Relat Metab Disord 2002; 26: 344–353.
Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 1999; 48: 94–98.
Xu X, De Pergola G, Eriksson PS, Fu L, Carlsson B, Yang S et al. Postreceptor events involved in the up-regulation of beta-adrenergic receptor mediated lipolysis by testosterone in rat white adipocytes. Endocrinology 1993; 132: 1651–1657.
De Pergola G . The adipose tissue metabolism: role of testosterone and dehydroepiandrosterone. Int J Obes Relat Metab Disord 2000; 24 (Suppl 2): S59–S63.
Björntorp P . Growth hormone, insulin-like growth factor-i and lipid metabolism: interactions with sex steroids. Horm Res 1996; 46: 188–191.
Xu XF, De Pergola G, Björntorp P . Testosterone increases lipolysis and the number of beta-adrenoceptors in male rat adipocytes. Endocrinology 1991; 128: 379–382.
Raheja KL, Reber EF . Relative ineffectiveness of exogenous as compared with endogenous testosterone in combating hypercholesterolemia. Hormone and metabolic research. Hormon- und Stoffwechselforschung. Horm Metab 1974; 6: 88.
Acknowledgements
We thank Lone Pridal, Annette Hansen, Michael Wilken, Lotte G Soerensen, Aase Vinterby, Nicolaj Steffensen, Frank Strauss and A-C Løff for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this work has been presented as a poster at the EASD conference 2004 (abstract no. 631).
Rights and permissions
About this article
Cite this article
Christoffersen, B., Raun, K., Svendsen, O. et al. Evalution of the castrated male Sprague–Dawley rat as a model of the metabolic syndrome and type 2 diabetes. Int J Obes 30, 1288–1297 (2006). https://doi.org/10.1038/sj.ijo.0803261
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803261
Keywords
This article is cited by
-
Mammalian models of diabetes mellitus, with a focus on type 2 diabetes mellitus
Nature Reviews Endocrinology (2023)
-
Surgical castration versus chemical castration in donkeys: response of stress, lipid profile and redox potential biomarkers
BMC Veterinary Research (2020)
-
Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice
Scientific Reports (2016)
-
Effect of testosterone deficiency on cholesterol metabolism in pigs fed a high-fat and high-cholesterol diet
Lipids in Health and Disease (2015)
-
Transcriptomic analysis of hepatic responses to testosterone deficiency in miniature pigs fed a high-cholesterol diet
BMC Genomics (2015)