Abstract
Objective:
High-carbohydrate (HC)–high-fibre diets are recommended for weight loss and for treating and preventing diseases such as diabetes and cardiovascular disease. We report a randomised trial comparing high-fat (HF) and high-protein (HP) diets with the conventional approach.
Research design and methods:
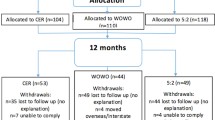
A total of 93 overweight insulin-resistant women received advice following randomisation to HF, HP or HC dietary regimes, to achieve weight loss followed by weight maintenance over 12 months. Weight, body composition and measures of carbohydrate and lipid metabolism were investigated.
Results:
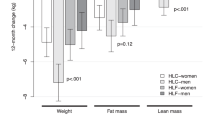
Retention rates were 93% for HP and 75% for HC and HF. Features of the metabolic syndrome improved in all groups during the first 6 months, to a greater extent on HF and HP than an HC. During the second 6 months the HF group had increases in waist circumference (mean difference 4.4 cm (95% CI 3.0, 5.8)), fat mass (2.3 kg (1.5, 3.1)), triglycerides (0.28 mmol/l (0.09, 0.46)) and 2 h glucose (0.70 mmol/l (0.22, 1.18)). Overall there was substantial sustained improvement in waist circumference, triglycerides and insulin in the HP group and sustained but more modest changes on HC. Dietary compliance at 12 months was poor in all groups.
Conclusions:
HP and HC approaches appear to be appropriate options for insulin-resistant individuals. When recommending HP diets appropriate composition of dietary fat must be ensured. HC diet recommendations must include advice regarding appropriate high-fibre, low glycaemic index foods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ministry of Health. Food and Nutrition Guidelines for Healthy Adults: A Background Paper. Ministry of Health, Wellington: New Zealand, October, 2003.
The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Recommendations for the nutritional management of patients with diabetes mellitus. Eur J Clin Nutr 2000; 54 (4): 353–355.
Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2003; 26 (Suppl 1): 51–61.
Atkins RC . Dr Atkins’ New Diet Revolution. Avon Books Inc.: New York, 1992.
Sears B . A Week in the Zone. 1st edn. Harper Collins Publishers Inc.: New York, 2000.
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003; 348 (21): 2082–2090.
Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003; 348 (21): 2074–2081.
Landers P, Wolfe MM, Glore S, Guild R, Phillips L . Effect of weight loss plans on body composition and diet duration. J Okla State Med Assoc 2002; 95 (5): 329–331.
Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA . A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab 2003; 88 (4): 1617–1623.
Parker B, Noakes M, Luscombe N, Clifton P . Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25 (3): 425–430.
Dumesnil JG, Turgeon J, Tremblay A, Poirier P, Gilbert M, Gagnon L et al. Effect of a low-glycaemic index–low-fat–high protein diet on the atherogenic metabolic risk profile of abdominally obese men. Br J Nutr 2001; 86 (5): 557–568.
Skov AR, Toubro S, Ronn B, Holm L, Astrup A . Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord 1999; 23 (5): 528–536.
Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP . Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005; 142 (6): 403–411.
Brehm BJ, Spang SE, Lattin BL, Seeley RJ, Daniels SR, D’Alessio DA . The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J Clin Endocrinol Metab 2005; 90 (3): 1475–1482.
Sargrad KR, Homko C, Mozzoli M, Boden G . Effect of high protein vs high carbohydrate intake on insulin sensitivity, body weight, hemoglobin A1c, and blood pressure in patients with type 2 diabetes mellitus. J Am Diet Assoc 2005; 105 (4): 573–580.
Luscombe-Marsh ND, Noakes M, Wittert GA, Keogh JB, Foster P, Clifton PM . Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am J Clin Nutr 2005; 81 (4): 762–772.
Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 2004; 140 (10): 778–786.
Due A, Toubro S, Skov AR, Astrup A . Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes 2004; 28 (10): 1283–1290.
Brinkworth GD, Noakes M, Parker B, Foster P, Clifton PM . Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventional weight-loss diet, in obese adults with Type 2 diabetes: one-year follow-up of a randomised trial. Diabetologia 2004; 47 (10): 1677–1686.
Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM . Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes 2004; 28 (5): 661–670.
Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ . Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005; 293 (1): 43–53.
McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia 2005; 48 (1): 8–16.
Hill AJ, Blundell JE . Macronutrients and satiety: the effects of a high-protein or high-carbohydrate meal on subjective motivation to eat and food preferences. Nutrition and Behavior 1986; 3: 133–144.
Fumaz CR, Tuldra A, Ferrer MJ, Paredes R, Bonjoch A, Jou T et al. Quality of life, emotional status, and adherence of HIV-1-infected patients treated with efavirenz versus protease inhibitor-containing regimens. J Acquir Immune Defic Syndr 2002; 29 (3): 244–253.
McNair DM, Lorr M, Dropppleman LF . EdITS Manual for the Profile of Mood States. Educational and Industrial Testing Service: San Diego, CA, 1992.
Arroll B, Jackson R, Beaglehole R . Validation of a three-month physical activity recall questionnaire with a seven-day food intake and physical activity diary. Epidemiology 1991; 2 (4): 296–299.
National Institute of Health. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr 1996; 64 (Suppl 3): 524–532.
McAuley KA, Williams SM, Mann JI, Goulding A, Chisholm A, Wilson N et al. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care 2002; 25 (3): 445–452.
Russell D, Parnell W, Wilson N . NZ Food: NZ People. Key results of the 1997 National Nutrition Survey. Ministry of Health, Wellington: New Zealand, August 1999.
Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L . XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27 (1): 155–161.
Acknowledgements
We are grateful to the participants; Glenys Henshaw, the study dietitian; Michelle Harper and Ashley Duncan, who undertook most of the laboratory analyses; Charla Hopkins, who was involved in the first 6 months of this study; and Victoria Farmer for research assistance. Funding was provided by the Health Research Council of New Zealand and in the initial stages of this study financial support was obtained from a Bristol Myers Squibb Mead Johnson Unrestricted Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McAuley, K., Smith, K., Taylor, R. et al. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obes 30, 342–349 (2006). https://doi.org/10.1038/sj.ijo.0803075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803075
Keywords
This article is cited by
-
Preoperative Carbohydrate Quality Index Is Related to Markers of Glucose Metabolism 12 Months After Roux-en-Y Gastric Bypass
Obesity Surgery (2023)
-
Water extract from processed Polygonum multiflorum modulate gut microbiota and glucose metabolism on insulin resistant rats
BMC Complementary Medicine and Therapies (2020)
-
Conversations between self and self as Sigmund Freud—A virtual body ownership paradigm for self counselling
Scientific Reports (2015)
-
Dietary protein and urinary nitrogen in relation to 6-year changes in fat mass and fat-free mass
International Journal of Obesity (2015)
-
Long-term adherence to the New Nordic Diet and the effects on body weight, anthropometry and blood pressure: a 12-month follow-up study
European Journal of Nutrition (2015)