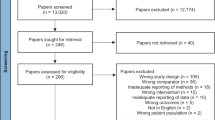
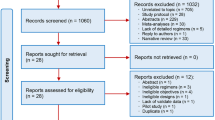
Abstract
The prevalence of obesity is increasing at an alarming rate and a plethora of complementary therapies are on offer claiming effectiveness for reducing body weight. The aim of this systematic review is to critically assess the evidence from randomized controlled trials (RCTs) and systematic reviews of complementary therapies for reducing body weight. Literature searches were conducted on Medline, Embase, Amed, and the Cochrane Library until January 2004. Hand-searches of relevant medical journals and bibliographies of identified articles were conducted. There were no restrictions regarding the language of publication. Trial selection, quality assessment and data abstraction were performed systematically and independently by two authors. Data from RCTs and systematic reviews, which based their findings on the results of RCTs, were included. Six systematic reviews and 25 additional RCTs met our inclusion criteria and were reviewed. The evidence related to acupuncture, acupressure, dietary supplements, homeopathy and hypnotherapy. Except for hypnotherapy, Ephedra sinica and other ephedrine-containing dietary supplements the weight of the evidence is not convincing enough to suggest effectiveness. For these interventions, small effects compared with placebo were identified. In conclusion, our findings suggest that for most complementary therapies, the weight of the evidence for reducing body is not convincing. Hypnotherapy, E. sinica and other ephedrine-containing dietary supplements may lead to small reductions in body weight. However, the intake of E. sinica and ephedrine is associated with an increased risk of adverse events. Interventions suggesting positive effects in single RCTs require independent replication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
World Health Organization. Obesity: preventing and managing the global epidemic. World Health Organization: Geneva; 1998.
National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity and health risk. Arch Intern Med 2000; 160: 898–904.
Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA . Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001; 161: 1581–1586.
Key TJ, Allen NE, Spencer EA, Travis RC . The effect of diet on risk of cancer. Lancet 2002; 360: 861–868.
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS . Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313.
Flegal KM, Carroll MD, Ogden CL, Johnson CL . Prevalence and trends in obesity among US adults 1999–2000. JAMA 2002; 288: 1723–1727.
National Audit Office. Tackling obesity in England. Stationery Office: London; 2001.
Seidell JC, Flegal KM . Assessing obesity: classification and epidemiology. Br Med Bull 1997; 53: 238–252.
Prentice AM, Jebb SA . Obesity in Britain: gluttony or sloth? BMJ 1995; 311: 437–439.
Heini AF, Weinsier RL . Divergent trends in obesity and fat intake patterns: the American paradox. Am J Med 1997; 102: 259–264.
Blanck HM, Khan LK, Serdula MK . Use of nonprescription weight loss products. JAMA 2001; 286: 930–935.
Jadad AR, Moore RA, Carrol D et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Controlled Clin Trials 1996; 17: 1–12.
Ernst E . Acupuncture/acupressure for weight reduction? Wien Klin Wochenschr 1997; 109: 60–62.
Mazzoni R, Mannucci E, Rizzello SM, Ricca V, Rotella CM . Failure of acupuncture in the treatment of obesity: a pilot study. Eating Weight Disord 1999; 4: 198–202.
Sun Qingfu. Simple obesity and obesity hyperlipidemia treated with otoacupoint pellet pressure and body acupuncture. J Trad Chin Med 1993; 13: 22–26.
Steiner RP, Kupper N, Davis AW . Obesity and appetite control: comparison of acupuncture therapies and behavior modification. Proceedings of the International Forum on Family Medicine Education, Society of Teachers of Family Medicine, Kansas City, MO 1983. pp 313–326.
Lacey JM, Terhakovec AM, Foster GD . Acupuncture for the treatment of obesity: a review of the evidence. Int J Obes Relat Metab Disord 2003; 27: 419–427.
Paranjpe P, Patki P, Patwardhan B . Ayurvedic treatment of obesity: a randomised double-blind, placebo-controlled clinical trial. J Ethnopharmacol 1990; 29: 1–11.
Kanauchi O, Deuchi K, Imasato Y, Shizukuishi M, Kobayashi E . Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci Biotech Biochem 1995; 59: 786–790.
Nauss JL, Thompson JL, Nagyuvary J . The binding of micellar lipids to chitosan. Lipids 1983; 18: 714–719.
Nagyvary JJ, Falk JD, Hill ML, Schmidt ML, Wilkins AK, Bradbury EL . The hypolipidemic activity of chitosan and other polysaccharides in rats. Nutr Rep Int 1979; 20: 677–684.
Vahouny GV, Satchithanandam S, Cassidy MM, Lightfood FB, Fzirda I . Comparative effects of chitosan and cholestyramine on lymphatic absorption of lipids in the rat. Am J Clin Nutr 1983; 38: 278–284.
Ernst E, Pittler MH . Chitosan as a treatment for body weight reduction? Perfusion 1998; 11: 461–465.
Wuolijoki E, Hirvelä T, Ylitalo P . Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find Exp Clin Pharmacol 1999; 21: 357–361.
Schiller RN, Barrager E, Schauss AG, Nichols EJ . A randomized, double-blind, placebo-controlled study examining the effects of a rapidly soluble chitosan dietary supplement on weight loss and body composition in overweight and mildly obese individuals. JANA 2001; 4: 42–49.
Pittler MH, Abbot NC, Harkness EF, Ernst E . Randomised, double blind trial of chitosan for body weight reduction. Eur J Clin Nutr 1999; 53: 379–381.
Ho SC, Tai ES, Eng PHK, Tan CE, Fok ACK . In the absence of dietary surveillance chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singapore Med J 2001; 42: 6–10.
Anderson RA . Effects of chromium on body composition and weight loss. Nutr Rev 1998; 56: 266–270.
Offenbacher EG, Pi-Sunyer FX . Chromium in human nutrition. Ann Rev Nutr 1988; 8: 543–563.
Mertz W . Chromium in human nutrition: a review. J Nutr 1993; 123: 626–633.
Anderson RA . Essentiality of chromium in humans. Sci Total Environ 1989; 86: 75–81.
Crawford V, Scheckenbach R, Preuss HG . Effects of niacin-bound chromium supplementation on body composition in overweight African-American women. Diabetes Obes Metab 1999; 1: 331–337.
Pittler MH, Stevinson C, Ernst E . Chromium picolinate for body weight reduction. Meta-analysis of randomized trials. Int J Obes Relat Metab Disord 2003; 27: 522–529.
Cowburn G, Hillsdon M, Hankey CR . Obesity management by life-style strategies. Br Med Bull 1997; 53: 389–408.
Blumenthal M, Goldberg A, Brinckmann J . Herbal medicine. expanded commission E monographs. American Botanical Council: Austin; 2000.
Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagne J . Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance. JAMA 2003; 289: 1537–1545.
Haller CA, Benowitz NL . Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. NEJM 2000; 343: 1833–1838.
Fontanarosa PB, Rennie D, DeAngelis CD . The need for regulation of dietary supplements – lessons from ephedra. JAMA 2003; 289: 1568–1570.
Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C . Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent. JAMA 1998; 280: 1596–1600.
Mattes RD, Bormann L . Effects of (−)-hydroxycitric acid on appetitive variables. Physiol Behav 2000; 71: 87–94.
Thom E . Hydroxycitrate (HCA) in the treatment of obesity. Int J Obes Relat Metab Disord 1996; 20 (Suppl 4): 75.
Doi K . Effect of konjac fibre (glucomannan) on glucose and lipids. Eur J Clin Nutr 1995; 49: S190–S197.
Walsh DE, Yaghoubian V, Behforooz A . Effect of glucomannan on obese patients: a clinical study. Int J Obes Relat Metab Disord 1983; 8: 289–293.
Pittler MH, Ernst E . Guar gum for body weight reduction. Meta-analysis of randomized trials. Am J Med 2001; 110: 724–730.
Nissen S, Panton L, Wilhelm R, Fuller Jr JC . Effect of β-hydroxy-β-methylbutyrate (HMB) supplementation on strength and body composition of trained and untrained males undergoing intense resistance training. FASEB J 1996B; 10: A287.
Vukovich MD, Stubbs NB, Bohlken RM, Desch MF, Fuller Jr JC, Rathmacher JA . The effect of dietary β-hydroxy-β-methylbutyrate (HMB) on strength gains and body composition changes in older adults. FASEB J 1997; 11: A376.
Capassso F, Gagniella TS, Grandolini G, Izzo AA . Phytotherapy. Springer: Berlin, Heidelberg; 2003.
Rodríguez-Morán M, Guerrero-Romero F, Laczano-Burciaga G . Lipid- and glucose-lowering efficacy of Plantago psyllium in type II diabetes. J Diabetes Comp 1998; 12: 273–278.
Stanko RT, Reynolds HR, Hoyson R, Janosky JE, Wolf R . Pyruvate supplementation of a low-cholesterol, low-fat diet: effects on plasma lipid concentrations and body composition in hyperlipidemic patients. Am J Clin Nutr 1994; 59: 423–427.
Stanko RT, Arch JE . Inhibition of regain of body weight and fat with addition of 3-carbon compounds to the diet with hyperenergetic refeeding after weight reduction. Int J Obese Relat Metabol Disord 1996; 20: 925–930.
Kalman D, Colker CM, Wilets I, Roufs JB, Antonio J . The effects of pyruvate supplementation on body composition in overweight individuals. Nutrition 1999; 15: 337–340.
Kalman D, Colker CM, Stark R, Minsch A, Wilets I, Antonio J . Effects of pyruvate supplementation on body composition and mood. Curr Therap Res 1998; 59: 793–802.
Kucio C, Jonderko K, Piskorska D . Does yohimbine act as a slimming drug? Isr J Med Sci 1991; 27: 550–556.
Sax L . Yohimbine does not affect fat distribution in men. Int J Obes Relat Metab Disord 1991; 15: 561–565.
Berlin I, Stalla-Bourdillon A, Thuillier Y, Turpin G, Puech J . Lack of efficacity of yohimbine in the treatment of obesity. J Pharmacol 1986; 17: 343–347.
Werk W, Galland F . Helianthus-tuberosus-Therapie bei Übergewicht. Therapiewoche 1994; 44: 34–39.
Schmidt JM, Ostermayr B . Does a homeopathic ultramolecular dilution of Thyroidinum 30cH affect the rate of body weight reduction in fasting patients? A randomized placebo-controlled double-blind clinical trial. Homeopathy 2002; 91: 197–206.
Allison DB, Faith MS . Hypnosis as an adjunct to cognitive-behavioral psychotherapy for obesity: a meta-analytical reappraisal. J Consult Clin Psychol 1996; 64: 513–516.
Kirsch I, Montgomery G, Sapirstein G . Hypnosis as an adjunct to cognitive-behavioral psychotherapy: a meta-analysis. J Consult Clin Psychol 1995; 63: 214–220.
Stradling J, Roberts D, Wilson A, Lovelock F . Controlled trial of hypnotherapy for weight loss in patients with obstructive sleep apnoea. Int J Obes Relat Metab Disord 1998; 28: 278–281.
Allison DB, Fontaine KR, Heshka S, Mentore JL, Heymsfield SB . Alternative treatments for weight loss: a critical review. Crit Rev Food Sci Nutr 2001; 41: 1–28.
Arterburn D, Hitchcock Noël P . Obesity. BMJ 2001; 322: 1406–1409.
Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL . Behavior therapy and sibutramine for the treatment of adolescent obesity. JAMA 2003; 289: 1805–1812.
Blanck HM, Khan LK, Serdula MK . Use of nonprescription weight loss products. Results from a multistate survey. JAMA 2001; 286: 930–935.
Egger G, Cameron-Smith D, Stanton R . The effectiveness of popular, non-prescription weight loss supplements. Med J Aust 1999; 171: 604–608.
Hosobuchi C, Rutanassee L, Bassin S-L, Wong N-D . Efficacy of acacia, pectin and guar gum-based fiber supplementation in the control of hypercholesterolaemia. Nutr Res 1999; 19: 643–649.
Najemnik C, Kritz H, Irsigler K et al. Guar and its effects on metabolic control in type II diabetic patients. Diabetes Care 1984; 7: 215–220.
Wirth A, Middlehoff G, Braeunig C, Schlierf G . Treatment of familial hypercholesterolaemia with a combinations of bezafibrate and guar. Atherosclerosis 1982; 45: 291–297.
Hänsel R, Keller K, Rimpler H, Schneider G (eds) Hagers Handbuch der pharmazeutischen Praxis. Springer: Berlin, Heidelberg; 1992.
Stearns DM, Wise JP, Patierno SR, Wetterhahn KE . Chromium(III) picolinate produces chromosome damage in Chinese hamster ovary cells. FASEB 1995; 9: 1643–1648.
Anderson RA, Bryden NA, Polansky NN . Lack of toxicity of chromium chloride and chromium picolinate in rats. J Am Coll Nutr 1997; 16: 273–279.
Kato I, Vogelman JH, Karkoszka J et al. Effect of supplementation with chromium picolinate on antibody titers to 5-hydroxymethyl uracil. Eur J Epidemiol 1998; 14: 621–626.
Hepburn DDD, Xiao J, Bindon S, Vincent JB, O’Donnell J . Nutritional supplement chromium picolinate causes sterility and lethal mutations in Drosophila melanogaster. PNAS 2003; 100: 3766–3771.
Martin WR, Fuller R . Suspected chromium picolinate-induced rhabdomyolysis. Pharmacotherapy 1998; 18: 860–862.
Scroggie DA, Harris M, Sakai L . Rhabdomyolysis associated with nutritional supplement use. J Clin Rheumatol 2000; 6: 328–332.
Cerulli J, Grabe DW, Gauthier I, Malone M, McGoldrick MD . Chromium picolinate toxicity. Ann Pharmacother 1998; 32: 428–431.
Young PC, Turiansky GW, Bonner MW, Benson PM . Acute generalized exanthematous pustulosis induced by chromium picolinate. J Am Acad Dermatol 1999; 41: 820–823.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest: none.
Funding: none external.
Rights and permissions
About this article
Cite this article
Pittler, M., Ernst, E. Complementary therapies for reducing body weight: a systematic review. Int J Obes 29, 1030–1038 (2005). https://doi.org/10.1038/sj.ijo.0803008
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803008
Keywords
This article is cited by
-
Acute assessment of subjective appetite and implicated hormones after a hypnosis-induced hallucinated meal: a randomized cross-over pilot trial
Reviews in Endocrine and Metabolic Disorders (2020)
-
Obesity and Clinical Riskiness Relationship: Therapeutic Management by Dietary Antioxidant Supplementation—a Review
Applied Biochemistry and Biotechnology (2015)
-
Randomized trial of tapas acupressure technique for weight loss maintenance
BMC Complementary and Alternative Medicine (2012)
-
A pilot study to evaluate the effect of Taeumjowi-tang on obesity in Korean adults: study protocol for a randomised, double-blind, placebo-controlled, multicentre trial
Trials (2012)