Abstract
OBJECTIVE:
To evaluate whether snacking would improve weight loss and weight maintenance in overweight individuals within the context of a structured meal replacement (MR) weight loss program.
DESIGN:
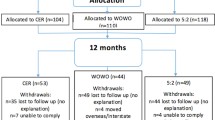
A prospective 24 week, 2 (snacking vs nonsnacking) × 2 (MR vs meal replacement augmented with snacks (MRPS)) randomized trial. Participants were instructed to limit their total daily intake to 1200 (women) or 1500 (men) kcals. Those receiving the MR program were instructed not to snack while those in the MRPS program were told to snack three times per day.
SUBJECTS:
A total of 100 participants were block-randomized, based on prestudy snacking status (high vs low), to receive a standard meal replacement program (MR) or MRPS.
MEASUREMENTS:
Weight, height, blood pressure, lipid fractions, glucose, and insulin were assessed at the baseline, 12-, and 24 weeks.
RESULTS:
Completers analysis at 24 weeks demonstrated a significant time effect (F(1,46)=44.6, P<0.001), indicating that all participants lost significant amounts of weight regardless of group assignment. An intention-to-treat model resulted in similar results. By week 24, the average weight loss across groups was 4.6 kg. There also were significant improvements across all groups among completers for systolic blood pressure (P=0.047), cholesterol (P=0.001), LDL (P=0.001), glucose (P=0.004), and insulin (P=0.001) at week 12, and glucose (P=0.001) and insulin at week 24 (P=0.003).
CONCLUSIONS:
Our results suggest that a participant's preferences for snacking did not affect their response to treatment. Snackers and nonsnackers responded equally well whether they received a standard meal replacement program or one augmented with snacks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Drummond S, Crombie N, Kirk T . A critique of the effects of snacking on body weight status. Eur J Clin Nutr 1996; 50: 779–783.
Bellisle F, McDevitt R, Prentice AM . Meal frequency and energy balance. Br J Nutr 1997; 77 (Suppl 1): S57–S70.
Fabry P, Fodor J, Hejl Z, Braun T, Zvolankova K . The frequency of meals: its relation to overweight, hypercholesterolaemia, and decreased glucose tolerance. Lancet 1964; ii: 614–615.
Fabry P, Hejda S, Cerny K, Osancova K, Pechar J . Effect of meal frequency in schoolchildren: changes in weight-height proportion and skinfold thickness. Am J Clin Nutr 1966; 18: 358–361.
Hejda S, Fabry P . Frequency of food intake in relation to some parameters of the nutritional status. Nutr Dieta 1964; 6: 216–221.
Kant AK, Schatzkin A, Graubard BI, Ballard-Barbash R . Frequency of eating occasions and weight change in the NHANES I Epidemiologic Follow-up Study. Int J Obes Relat Metab Disord 1995; 19: 468–474.
Ma Y, Bertone ER, Stanek 3rd EJ, Reed GW, Hebert JR, Cohen NL, Merriam PA, Ockene IS . Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003; 158: 85–92.
Titan SMO, Bingham S, Welch A, Luben R, Oakes S, Day N, Khaw KT . Frequency of eating and concentrations of serum cholesterol in the Norfolk population of the European prospective investigation into cancer (EPIC-Norfolk): cross sectional study. BMJ 2001; 323: 1–5.
Cunnane SC, Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Venketeshwer R, Jenkins AL, Buckley G, Patten R, Singer W, Corey P, Josse R . Nibbling versus gorging: Metabolic advantages of increased meal frequency. N Engl J Med 1989; 321: 929–934.
Edelstein SL, Barrett-Connor EL, Wingard DL, Cohn BA . Increased meal frequency associated with decreased cholesterol concentrations. Am J Clin Nutr 1992; 55: 664–669.
McGrath SA, Gibney MJ . The effects of altered frequency of eating on plasma lipids in free-living healthy males on normal self-selected diets. Eur J Clin Nutr 1994; 48: 402–407.
Ashley JM, St Jeor ST, Schrage JP, Perumean-Chaney SE, Gilbertson MC, McCall NL, Bovee V . Weight control in the physician's office. Arch Intern Med 2001; 161: 1599–1604.
Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G . Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr 1999; 69: 198–204.
Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G . Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res 2000; 8: 399–402.
Heymsfield SB, van Mierlo CAJ, van der Knaap HCM, Heo M, Frier HI . Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003; 27: 537–549.
Rothacker DQ . Five-year self-management of weight using meal replacements: comparison with matched controls in rural Wisconsin. Nutrition 2000; 16: 344–348.
Noakes M, Foster PR, Keogh JB, Clifton PM . Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr 2004; 134: 1894–1899.
Teixeira PJ, Going SB, Sardinha LB, Lohman TG . A review of psychosocial pre-treatment predictors of weight control. Obes Rev 2005; 6: 43–65.
National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity: the evidence report. US Government Press: Washington, DC; 1998.
Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT Statement. Revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001; 285: 1987–1991.
Shadish WR, Cook TD, Campbell DT . Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton-Mifflin; 2002.
Hampl JS, Heaton CLB, Taylor CA . Snacking patterns influence energy and nutrient intakes but not body mass index. J Hum Nutr Diet 2003; 16: 3–11.
Arnold L, Ball MJ, Duncan AW, Mann J . Effect of isoenergetic intake of three or nine meals on plasma lipoproteins and glucose metabolism. Am J Clin Nutr 1993; 46: 611–620.
McManus K, Antinoro L, Sacks F . A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord 2001; 25: 1503–1511.
Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L . A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 348; 21: 2074–2081.
Acknowledgements
This study was funded by a grant from the Slim Fast Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poston, W., Haddock, C., Pinkston, M. et al. Weight loss with meal replacement and meal replacement plus snacks: a randomized trial. Int J Obes 29, 1107–1114 (2005). https://doi.org/10.1038/sj.ijo.0803007
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803007
Keywords
This article is cited by
-
Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: a randomised trial
European Journal of Clinical Nutrition (2016)
-
Effectiveness of a Medifast meal replacement program on weight, body composition and cardiometabolic risk factors in overweight and obese adults: a multicenter systematic retrospective chart review study
Nutrition Journal (2015)
-
Eating frequency is inversely associated with blood pressure and hypertension in Korean adults: analysis of the Third Korean National Health and Nutrition Examination Survey
European Journal of Clinical Nutrition (2014)
-
Popcorn is more satiating than potato chips in normal-weight adults
Nutrition Journal (2012)