Abstract
CONTEXT: Chitosan, a deacetylated chitin, is a widely available dietary supplement purported to decrease body weight and serum lipids through gastrointestinal fat binding. Although evaluated in a number of trials, its efficacy remains in dispute.
OBJECTIVE: To evaluate the efficacy of chitosan for weight loss in overweight and obese adults.
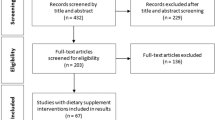
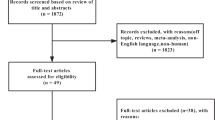
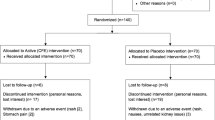
DESIGN AND SETTING: A 24-week randomised, double-blind, placebo-controlled trial, conducted at the University of Auckland between November 2001 and December 2002.
PARTICIPANTS: A total of 250 participants (82% women; mean (s.d.) body mass index, 35.5 (5.1) kg/m2; mean age, 48 (12) y)
INTERVENTIONS: Participants were randomly assigned to receive 3 g chitosan/day (n=125) or placebo (n=125). All participants received standardised dietary and lifestyle advice for weight loss. Adherence was monitored by capsule counts.
MAIN OUTCOME MEASURES: The primary outcome measure was change in body weight. Secondary outcomes included changes in body mass index, waist circumference, body fat percentage, blood pressure, serum lipids, plasma glucose, fat-soluble vitamins, faecal fat, and health-related quality of life.
RESULTS: In an intention-to-treat analysis with the last observation carried forward, the chitosan group lost more body weight than the placebo group (mean (s.e.), −0.4 (0.2) kg (0.4% loss) vs +0.2 (0.2) kg (0.2% gain), P=0.03) during the 24-week intervention, but effects were small. Similar small changes occurred in circulating total and LDL cholesterol, and glucose (P<0.01). There were no significant differences between groups for any of the other measured outcomes.
CONCLUSION: In this 24-week trial, chitosan treatment did not result in a clinically significant loss of body weight compared with placebo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sugano M, Fujikawa T, Hiratsuji Y, Nakashima K, Fukuda N, Hasegawa Y . A novel use of chitosan as a hypocholesterolemic agent in rats. Am J Clin Nutr 1980; 33: 787–793.
Zacour AC, Silva ME, Cecon PR, Bambirra EA, Vieira EC . Effect of dietary chiton on cholesterol absorption and metabolism in rats. J Nutr Sci Vitaminol 1992; 38: 609–613.
Deuchi K, Kanauchi O, Imasato Y, Kobayashi E . Effect of the viscosity or deacetylation degree of chitosan on fecal fat excreted from rats fed on a high fat diet. Biosci Biotech Biochem 1995; 59: 781–785.
Nagyvary JJ, Falk JD, Hill ML, Schmidt ML, Wilkins AK, Brdbury EL . The hypolipidemic activity of chitosan and other polysaccharides in rats. Nutr Rep Int 1979; 30: 677–684.
Ormrod DJ, Holmes CC, Miller TE . Dietary chitosan inhibits hypercholesterolaemia and atherogenesis in the apolipoprotein E-deficient mouse model of atherosclerosis. Atherosclerosis 1998; 138: 329–334.
Schiller RN, Barrager E, Schauss AG, Nichols EJ . A randomized, double-blind, placebo-controlled study examining the effects of a rapidly soluble Chitosan dietary supplement on weight loss and body composition in overweight and mildly obese individuals. J Am Nutraceut Assoc 2001; 4: 42–49.
Zahorska-Markiewicz B, Krotkiewski M, Olszanecka-Glinianowicz M, Zurakowski A . Effect of chitosan in the complex treatment of obesity. Polish Med J 2002; 74: 129–132.
Ernst E, Pittler MH . Chitosan as a treatment for body weight reduction? A meta-analysis. Perfusion 1998; 11: 461–465.
Ho SC, Tai ES, Eng PHK, Tan CE, Fok ACK . In the absence of dietary surveillance, Chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Sing Med J 2001; 42: 6–10.
Pittler MH, Abbot NC, Harkness EF, Ernst E . Randomized, double-blind trial of chitosan for body weight reduction. Eur J Clin Nutr 1999; 53: 379–381.
Allison DB, Fontaine KR, Heshka S, Mentore JL, Heymsfield SB . Alternative treatments for weight loss: a critical review. Crit Rev Food Sci Nutr 2001; 41: 1–28.
Egger G, Cameron-Smith D, Stanton R . The effectiveness of popular, non-prescription weight loss supplements. Med J Austr 1999; 171: 604–608.
Russell DG, Wilson NC . Life in New Zealand Commission Report, Volume 1: Executive overview. University of Otago: Dunedin; 1991.
Ware JE, Kosinski M, Keller SD . SF-36 physical and mental health summary scales: a users manual, 2nd edn. The Health Institute, New England Medical Centre: Boston, MA; 1994.
Lavik NJ, Clausen SE, Pedersen W . Eating behaviour, drug use, psychopathology and parental bonding in adolescents in Norway. Acta Psychiatr Scand 1991; 84: 387–390.
Schouten HJA . Planning group sizes in clinical trials with a continuous outcome and repeat measures. Statist Med 1999; 18: 255–264.
Frison L, Pocock SJ . Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Statist Med 1992; 11: 1685–1704.
Hand DJ, Crowder MJ . Practical longitudinal data analysis. Chapman and Hall: London; 1996.
Girola M, De Bernardi M, Contos S, Tripodi S, Ventura P, Guarino C, Marletta M . Dose effect in lipid-lowering activity of new dietary integrator (chitosan, garcinia cambogia extract and chrome). Acta Toxicol Ther 1996; 17: 25–40.
Wuolijoki E, Hirvela T, Ylitalo P . Decrease in serum LDL cholesterol with microcrystalline Chitosan. Methods Findings in Exp Clin Pharmacol 1999; 21: 357–361.
Bokura H, Kobayashi S . Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 2003; 57: 721–725.
Dunshea-Mooij C, Ni Mhurchu C, Bennett D, Rodgers A . Chitosan for overweight and obesity (Protocol). The Cochrane library, Issue 4. Update Software: Oxford; 2002.
Guerciolini R, Radu-Radulescu L, Boldrin M, Dallas J, Moore R . Comparative evaluation of fecal fat excretion induced by orlistat and chitosan. Obes Res 2001; 9: 364–367.
Gades MD, Stern JS . Chitosan supplementation and fecal fat excretion in men. Obes Res 2003; 11: 683–688.
Acknowledgements
This study could not have been conducted without the dedication of Human Nutrition Unit staff (Jane Easton (Study Manager), Santuri Rungan, Chao-Yuan Chen, David Anderson, Laura Gerulitis, Pia Nielson, Jeannette Eis, Cathelijne Reincke, Shannon McCarthy) and staff at the Clinical Trials Research Unit involved in programming (Alex Bormans, Barry Gray, Donovan Marshall, Clark Mills, Colleen Ng, Michael Ng, Jaco van Rooyen), data management (Michelle Barlow, Yvonne Cleverley, Terry Holloway, Daphne Hukui, Amanda Milne, Ellen Rhyno, Marissa Te Whui, Esther Vao, Sandhya Waghulde, Alison Young) and administrative support (Mary Cosson, Deanne Douglas, Sheila Fisher, Elizabeth Hawthorne). We would like to thank our Data Safety and Monitoring Committee (Katrina Sharples and Jim Mann), and we are particularly grateful for the contribution of the 250 ECHO study participants. This was an investigator-initiated study funded predominantly by the Health Research Council of New Zealand. Study treatments and funding for vitamin analyses was provided by Healtheries of New Zealand Ltd. CNM and AR held fellowships from the National Heart Foundation of New Zealand.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Mhurchu, C., Poppitt, S., McGill, AT. et al. The effect of the dietary supplement, Chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. Int J Obes 28, 1149–1156 (2004). https://doi.org/10.1038/sj.ijo.0802693
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802693
Keywords
This article is cited by
-
The effects of chitosan supplementation on anthropometric indicators of obesity, lipid and glycemic profiles, and appetite-regulated hormones in adolescents with overweight or obesity: a randomized, double-blind clinical trial
BMC Pediatrics (2022)
-
Efficacy of dietary supplements containing isolated organic compounds for weight loss: a systematic review and meta-analysis of randomised placebo-controlled trials
International Journal of Obesity (2021)
-
Chitosan modifies glycemic levels in people with metabolic syndrome and related disorders: meta-analysis with trial sequential analysis
Nutrition Journal (2020)
-
Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers
Applied Biochemistry and Biotechnology (2017)
-
A single oral dose of a polyglucosamine influences the bioavailability of [9-14C]-Oleic acid in adult female Göttingen minipigs
BMC Obesity (2016)