Abstract
OBJECTIVE: The primary goal of this study was to assess whether increases in fat-free mass (FFM) and decreases in total and percentage fat mass from 15 weeks of twice weekly supervised strength training would be maintained over 6 months of unsupervised exercise in a randomized controlled trial.
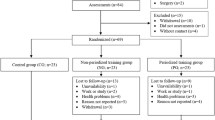
DESIGN: In all, 60 women aged 30–50 y, body mass index between 20 and 35 kg/m2, were randomized to control or treatment groups. The treatment group performed twice-weekly supervised strength training followed by 6 months of unsupervised training. Measurements at baseline, 15, and 39 weeks included body weight and body composition by dual-energy X-ray absorptiometry. Repeated measures regression was used to assess between-group differences for changes over time.
RESULTS: Almost 90% of prescribed exercise sessions were completed. The body composition treatment effects over 15 weeks were largely maintained over 6 months of unsupervised exercise. Over the total 39 weeks of strength training, the treatment group gained +0.89 kg more in FFM, lost −0.98 kg more in fat mass, and lost –1.63% more in percent body fat when compared to the control group. P-values for these between-group differences in 39-week changes were 0.009, 0.06, and 0.006, respectively. Strength training did not result in any significant weight loss or waist circumference attenuation. Adjustment for changes in energy intake and physical activity did not alter these results.
CONCLUSIONS: Twice-weekly strength training is behaviorally feasible for busy midlife women and the favorable body composition changes resulting from supervised strength training can be maintained over time. These findings lay the groundwork for determining the long-term health benefits of this behaviorally feasible exercise prescription, potentially including prevention of age-associated fat gains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL . Overweight and obesity in the United States: prevalence and trends, 1960–1964. Int J Obes Relat Metab Disord 1998; 22: 39–47.
Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO . A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr 1997; 66: 239–246.
Jeffery RW, Epstein LH, Wilson GT, Drewnowski A, Stunkard AJ, Wing RR . Long-term maintenance of weight loss: current status. Health Pyschol 2000; 19 (Suppl 1): 5–16.
US Department of Agriculture, Agricultural Research Service, Dietary Guidelines Advisory Committee. Report of the dietary guidelines advisory committee on the dietary guidelines for Americans, 1995, to the Secretary of Health and Human Services and the Secretary of Agriculture. 1995.
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. Division of Noncommunicable Diseases, Programme of Nutrition Family and Reproductive Health: Geneva, 1997.
McDowell MA, Briefel RR, Alaimo K, Bischof AM, Caughman CR, Carroll MD, Loria CM, Johnson CL . Energy and macronutrient intakes of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. In: Advance data from vital and health statistics; No 255. National Center for Health Statistics: Hyattsville, MD, 1994.
US Department of Health and Human Services. Physical activity and health: A Report of the Surgeon General. Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion: Atlanta, GA; 1996. pp 85–259.
DiPietro L . Physical activity, body weight, and adiposity: an epidemiologic perspective. Exerc Sport Sci Rev 1995; 23: 275–303.
DiPietro L . Physical activity in the prevention of obesity: current evidence and research issues. Med Sci Sports Exerc 1999; 31 (Suppl 11): S542–S546.
Schmitz KH, Jacobs DR, Leon AS, Schreiner PJ, Sternfeld B . Physical activity and body weight: associations over ten years in the CARDIA study. Int J Obes Relat Metab Disord 2000; 24: 1475–1487.
Schmitz KH, Jeffery RW . Public health interventions for prevention and treatment of obesity. Med Clin North Am 2000; 84: 491–512.
Schmitz KH, Jeffery RW . Obesity Prevention. In: Wadden TA, Stunkard AJ (eds). Obesity: theory and therapy, 3rd edn. New York, NY; Guilford Press, 2002. pp 556–593.
National Institutes of Health; National Heart, Lung, and Blood Institute. Obesity Education Initiative Expert Panel. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res 1998; 6 (Suppl 2): 51S–210S.
Pronk NP, Wing RR . Physical activity and long-term maintenance of weight loss. Obes Res 1994; 2: 587–599.
Fleck SJ, Kraemer WJ . Designing resistance training programs. 2nd edn. Human Kinetics: Champaign, IL. 1997.
Vaughan L, Zurlo F, Ravussin E . Aging and energy expenditure. Am J Clin Nutr 1991; 53: 821–825.
Keys A, Taylor HL, Grande F . Basal metabolism and age of adult man. Metabolism 1987; 22: 5979–5987.
Calloway DH, Zanni E . Energy requirements and energy expenditure of elderly men. Am J Clin Nutr 1980; 33: 2088–2092.
Tzankoff SP, Norris AH . Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol 1977; 43: 1001–1106.
Going S, Williams D, Lohman T . Aging and body composition: biological changes and methodological issues. Exerc Sport Sci Rev 1995; 23: 411–458.
Broeder CE, Burrhus KA, Svanevik LS, Wilmore JH . The effects of either high-intensity resistance or endurance training on resting metabolic rate. Am J Clin Nutr 1992; 55: 802–810.
Campbell WW, Crim MC, Young VR, Evans WJ . Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr 1994; 60: 167–175.
Chilibeck PD, Calder A, Sale DG, Webber CE . Twenty weeks of weight training increases lean tissue mass but not bone mineral mass or density in healthy, active young women. Can J Physiol Pharmacol 1996; 74: 1180–1185.
Cullinen K, Caldwell M . Weight training increases fat-free mass and strength in untrained young women. J Am Diet Assoc 1998; 98: 414–418.
Nelson ME, Fiatarone MA, Morganti CM, Tric I, Greenberg RA, Evans WJ . Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. JAMA 1994; 272: 1909–1914.
Nichols JF, Omizo DK, Peterson KK, Nelson KP . Efficacy of heavy-resistance training for active women over sixty: muscular strength body composition, and program adherence. JAGS 1993; 41: 205–210.
Weinsier RL, Schutz Y, Bracco D . Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr 1992; 55: 790–794.
Ballor DL, Harvey-Berino JR, Ades PA, Cryan J, Calles-Escandon J . Contrasting effects of resistance and aerobic training on body composition and metabolism after diet-induced weight loss. Metabolism 1996; 45: 179–183.
Ryan AS, Pratley RE, Elahi D, Goldberg AP . Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol 1995; 79: 818–823.
Taaffe DR, Pruitt L, Reim J, Butterfield G, Marcus R . Effect of sustained resistance training on basal metabolic rate in older women. J Am Geriatr Soc 1995; 43: 465–471.
Treuth MS, Hunter GR, Weinsier RL, Kell SH . Energy expenditure and substrate utilization in older women after strength training: 24-h calorimeter results. J Appl Physiol 1995; 78: 2140–2146.
Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE . Free-living activity energy expenditure in women who are successful and unsuccessful in maintaining a normal body weight. Am J Clin Nutr 2002; 75: 499–504.
McPherron AC, Lee SJ . Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 2002; 109: 595–601.
Donnelly JE, Jacobsen DJ, Jakicic JM, Whatley JE . Very low calorie diet with concurrent versus delayed and sequential exercise. Int J Obes Relat Metab Disord 1994; 18: 469–475.
Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM . Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females. Am J Clin Nutr 1991; 54: 56–61.
Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, Wilk J, Weinstock R, Buckenmeyer P, Berkowitz RI, Steen SN . Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol 1997; 65: 269–277.
Ballor DL, Katch VL, Becque MD, Marks CR . Resistance weight training during caloric restriction enhances lean body weight maintenance. Am J Clin Nutr 1988; 47: 19–25.
Marks BL, Ward A, Morris DH, Castellani J, Rippe JM . Fat-free mass is maintained in women following a moderate diet and exercise program. Med Sci Sports Exerc 1995; 27: 1243–1251.
Jakicic JM, Winters C, Lang W, Wing RR . Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA 1999; 282: 1554–1560.
PAR-Q Validation Report, British Columbia Ministry of Health, May 1978.
Ainsworth BE, LaMonte MJ, Whitt MC, Irwin ML, Drowatzky K . Development and validation of a physical activity questionnaire to assess moderate intensity activity in minority women, ages 40 and older. Proceedings of the NIH Women's Health Community Research Conference. Poster presented at the NIH Women's Health Community Research Conference, October 2000, Bethesda, MD.
Pereira MA, FitzGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM . A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc 1997; 29 (Suppl 6): S1–S205.
Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW . Assessment of body composition with use of dual-energy X-ray absorptiometry: evaluation and comparison with other methods. Mayo Clinic Proc 1993; 68: 867–873.
Wang J, Russell M, Mazariegos M, Burastero S, Thornton J, Lichtman S, Heymsfield SB, Pierson RN . Body fat by dual photon absorptiometry: comparisons with traditional methods in Asians, blacks and whites. Am J Hum Biol 1992; 4: 501–510.
Ross R, Rissanen J, Hudson R . Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord 1996; 20: 533–538.
Ross R, Janssen I . Physical activity, total and regional adiposity: dose–response considerations. Med Sci Sports Exerc 2001; 33 (Suppl 6): S521–S527.
Ross R, Janssen I . Is abdominal fat preferentially reduced in response to exercise induced weight loss? Med Sci Sports Exerc 1999; 31 (Suppl 11): S568–S572.
Ryan AS, Pratley RE, Goldberg AP, Elahi D . Resistive training increases insulin action in postmenopausal women. J Gerontol 1996; 51A: M199–M205.
Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA . Effects of resistance-training and endurance training on insulin sensitivity in nonobese, young women: a controlled, randomized trial. J Clin Endocrinol Metab 2000; 85: 2463–2468.
Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ . Exercise training and nutritional supplementation for physical frailty in very elderly people. New Engl J Med 1994; 330: 1769–1775.
Acknowledgements
This study was supported by a Minnesota Obesity Center pilot and feasibility grant (NIH Grant DK50456 from the National Institute of Diabetes and Digestive and Kidney Diseases) and the University of Minnesota GCRC (M01-RR00400). The investigators are grateful to the participants for their time and energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitz, K., Jensen, M., Kugler, K. et al. Strength training for obesity prevention in midlife women. Int J Obes 27, 326–333 (2003). https://doi.org/10.1038/sj.ijo.0802198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802198
Keywords
This article is cited by
-
Effects of resistance training on oxidative stress-related biomarkers in metabolic diseases: a review
Sport Sciences for Health (2018)
-
Acceptability of delivery modes for lifestyle advice in a large scale randomised controlled obesity prevention trial
BMC Public Health (2015)
-
The effect of physical exercise on postpartum fitness, hormone and lipid levels: a randomized controlled trial in primiparous, lactating women
Archives of Gynecology and Obstetrics (2015)
-
Outcomes of a 12-Month Web-Based Intervention for Overweight and Obese Men
Annals of Behavioral Medicine (2011)
-
Group versus individual cognitive-behavioral treatment for obesity: Results after 36 months
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2007)