Abstract
BACKGROUND: Previous studies have indicated that the secretion of the intestinal satiety hormone glucagon-like peptide-1 (GLP-1) is attenuated in obese subjects.
OBJECTIVE: To compare meal-induced response of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) in obese and lean male subjects, to investigate the effect of a major weight reduction in the obese subjects, and to look for an association between these hormones and ad libitum food intake.
METHOD: Plasma concentrations of intestinal hormones and appetite sensations were measured prior to, and every 30 min for 180 min after, ingestion of a 2.5 MJ solid test meal. Gastric emptying was estimated scintigraphically. An ad libitum lunch was served 3 h after the test meal.
SUBJECTS: Nineteen non-diabetic obese (body mass index (BMI) 34.1–43.8 kg/m2) and 12 lean (BMI 20.4–24.7 kg/m2) males. All obese subjects were re-examined after a mean stabilised weight loss of 18.8 kg (95% CI 14.4–23.2).
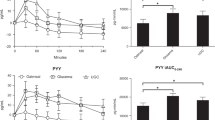
RESULTS: Total area under the GLP-1 response curve (AUCtotal, GLP-1) was lower in obese before and after the weight loss compared to lean subjects (P<0.05), although weight loss improved the response from 80 to 88% of that of the lean subjects (P=0.003). The GIP response was similar in obese and lean subjects. However, after the weight loss both AUCtotal, GIP and AUCincremental, GIP were lowered (P<0.05). An inverse correlation was observed between AUCincremental, GIP and energy intake at the subsequent ad libitum meal in all groups. In lean subjects ad libitum energy intake was largely predicted by the insulin response to the preceding meal (r2=0.67, P=0.001).
CONCLUSION: Our study confirmed previous findings of a reduced postprandial GLP-1 response in severely obese subjects. Following weight reduction, GLP-1 response in the obese subjects apparently rose to a level between that of obese and lean subjects. The data suggests that postprandial insulin and GIP responses are key players in short-term appetite regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Read NW . Role of gastrointestinal factors in hunger and satiety in man Proc Nutr Soc 1992 51: 7–11.
Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ . Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic function in man Dig Dis Sci 1993 38: 665–673.
Schjoldager BT, Mortensen PE, Christiansen J, Ørskov C, Holst JJ . GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans Dig Dis Sci 1989 34: 703–708.
Layer P, Holst JJ, Grandt D, Goebell H . Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans Dig Dis Sci 1995 40: 1074–1082.
Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C . Glucagon-like peptide-1: a potent regulator of food intake in humans Gut 1999 44: 81–86.
Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Rössner S, Hellström . Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men Int J Obes Relat Metab Disord 1999 23: 304–311.
Flint A, Raben A, Astrup A, Holst JJ . Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans J Clin Invest 1998 101: 515–520.
Flint A, Raben A, Holst JJ, Astrup A . Glucagon-like peptide-1 suppresses energy expenditure in obese humans Int J Obes Relat Metab Disord 1999 23: S35.
Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR . A role for glucagon-like peptide-1 in the central regulation of feeding Nature 1996 379: 69–72.
Ørskov C, Poulsen SS, Møller M, Holst JJ . Glucagon-like peptide 1 receptors in the subfornical organ and area postrema are accessible to circulating glucagon-like peptide-1 Diabetes 1996 45: 832–835.
Näslund E, Grybäck P, Backman L, Jacobsson H, Holst JJ, Thero-dorsson E, Hellström PM . Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying Dig Dis Sci 1998 43: 945–952.
Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V . Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 1996 38: 916–919.
Ranganath L, Norris F, Morgan L, Wright J, Marks V . Inhibition of carbohydrate-mediated glucagon-like peptide-1 (7-36)amide secretion by circulating non-estrified fatty acids Clin Sci 1999 96: 335–342.
Holst JJ, Schwartz TW, Lovgreen NA, Pedersen O, Beck-Nielsen H . Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity Int J Obes 1983 7: 529–538.
Holst JJ, Sørensen TIA, Andersen AH, Stadil F, Andersen B . Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio Scand J Gastroenterol 1979 14: 205–207.
Raben A, Andersen HB, Christensen NJ, Madsen J, Holst JJ, Astrup A . Evidence for an abnormal postprandial response to a high-fat meal in women predisposed to obesity Am J Physiol 1994 267: E549–559.
Raben A, Tagliabue A, Christensen NJ, Madsen J, Holst JJ, Astrup A . Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety Am J Clin Nutr 1994 60: 544–551.
Raben A, Andersen K, Karberg MA, Holst JJ, Astrup A . Acetylation of or β-cyclodextrin addition to potato starch: beneficial effect on glucose metabolism and appetite sensation Am J Clin Nutr 1997 66: 304–314.
Speechly DP, Buffenstein R . Appetite dysfunction in obese males: evidence for role of hyperinsulinaemia in passive overconsumption with a high fat diet Eur J Clin Nutr 2000 54: 225–233.
Holt SHA, Brand Miller JC, Petocz P . Interrelationship among postprandial satiety, glucose and insulin responses and changes in subsequent food intake Eur J Clin Nutr 1996 50: 788–797.
Fieseler P, Bridenbaugh S, Nustede R, Martell J, Ørskov C, Holst JJ, Nauck MA . Physiological augmentation of amino acid-induced insulin secretion by GIP and GLP-1 but not by CCK-8 Am J Physiol 1995 268: E949–955.
Verdich C, Lysgård Madsen J, Toubro S, Buemann B, Holst JJ, Astrup A . Effects of obesity and major weight reduction on gastric emptying Int J Obes Relat Metab Disord 2000 24: 899–905.
Krarup T, Madsbad S, Moody AJ, Regeur L, Faber OK, Holst JJ, Sestoft L . Diminished gastric inhibitory polypeptide (GIP) response to a meal in newly diagnosed type I (insulin dependent) diabetics J Clin Endocrinol Metab 1983 56: 1306–1312.
Ørskov C, Rabenhøj L, Kofod H, Wettergren A, Holst JJ . Production and secretion of amidated and glycine-extended glucagon-like peptide-1 (GLP-1) in man Diabetes 1994 43: 535–539.
Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ . Glucagon-like peptide-1 under goes differential tissue-specific metabolism in the anaesthetised pig Am J Physiol 1996 271: E458–E464.
Ranganath L, Norris F, Morgan L, Wright J, Marks V . The effect of circulating non-esterified fatty acids on the entero-insular axis Eur J Clin Invest 1999 29: 27–32.
Sarson DL, Kopelman PG, Besterman HS, Pilkington TRE, Bloom SR . Disparity between glucose-dependent insulinotropic polypeptide and insulin response in obese man Diabetologia 1983 25: 386–391.
Jones IR, Owens DR, Luzio SD, Hayes TM . Obesity is associated with increased post-prandial GIP levels which are not reduced by dietary restriction and weight loss Diabetes Metab (Paris) 1989 15: 11–22.
Ebert R, Creutzfeldt W . Gastric inhibitory polypeptide (GIP) hypersecretion in obesity depends on meal size and is not related to hyperinsulinemia Acta Diabetol Lat 1992 26: 1–15.
Groop PH . The influence of body weight, age and glucose tolerance on the relationship between GIP secretion and beta-cell function in man Scand J Clin Lab Invest 1989 49: 367–379.
Morgan LM, Tredger JAT, Hampton SM, French AP, Peake JCF, Marks V . The effect of dietary modification and hyperglycaemia on gastric emptying and gastric inhibitory polypeptide (GIP) secretion Br J Nutr 1988 60: 29–37.
Mazzaferri EL, Starich GH, Jeor ST . Augmented gastric inhibitory polypeptide and insulin response to a meal after an increase in carbohydrate (sucrose) intake J Clin Endocrinol Metab 1984 58: 640.
Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fendselau S, Schrezenmeir J, Hermansen K . Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects Am J Clin Nutr 1999 69: 1135–1143.
Cunningham K, Daly J, Horowitz M, Read NW . Gastrointestinal adaptation to diet of differing fat composition in human volunteers Gut 1991 32: 483–486.
Beck A, Villaume C, Bau HM, Garuot P, Chayvialle JA, Desalme A, Debray G . Long term influence of a wheat-bran supplemented diet on secretion of gastrointestinal hormones and on nutrient absorption in healthy man Hum Nutr Clin Nutr 1986 40C: 25–33.
Reimer RA, McBurney MI . Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats Endocrinology 1996 137: 3948–3956.
Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, Lillioja S, Bogardus C, Ravussin E . Insulin resistance associated with lower rates of weight gain in Pima indians J Clin Invest 1991 88: 168–173.
Chapman IM, Goble EA, Wittert GA, Morley JE, Horowitz M . Effect of intravenous glucose and euglycemic insulin infusion on short-term appetite and food intake Am J Physiol 1998 274: R596–603.
Woods SC, Lotter EC, Mckay LD, Porter D Jr . Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons Nature 1979 282: 503–505.
Deacon CF, Johnsen AH, Holst JJ . Degradation of glucagon-like peptide-1 by human plasma in vitro Yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo J Clin Endocrinol Metab 1995 80: 952–957.
Balkes HJ, Holst JJ, Mühler A, Brabrant G . Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors J Clin Endocrinol Metab 1997 85: 786–790.
Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A . Vagal hepatopancreatic reflex effects evoked by intraportal appearance of tGLP-1 Am J Physiol 1996 271: E808–813.
Hansen L, Deacon CF, Ørskov C, Holst JJ . Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine Endocrinology 1999 141: 5356–5363.
Acknowledgements
The authors wish to thank Jens Bülow, Lene Simonsen and Ingrid Thorn at the Department of Clinical Physiology, Bispebjerg Hospital, Copenhagen and Martin Kreutzer at the Research Department of Human Nutrition for performing the DEXA-scannings. We also wish to thank Inge Timmermann, Jannie M Larsen, Kirsten B Rasmussen, Bente Knap, Lars Paaske, Ulla Pedersen, Anette Vedelspang and Charlotte Kostecki for expert technical assistance.
This study was supported by the Danish Medical Research Council. The low calorie formula diet GERLINÉA® was donated by WASABRØD A/S; Skovlunde; Denmark.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verdich, C., Toubro, S., Buemann, B. et al. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes 25, 1206–1214 (2001). https://doi.org/10.1038/sj.ijo.0801655
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801655
Keywords
This article is cited by
-
Strategie di mantenimento del calo ponderale nel paziente con obesità
L'Endocrinologo (2023)
-
Dapagliflozin plus exenatide on patients with type 2 diabetes awaiting bariatric surgery in the DEXBASU study
Scientific Reports (2022)
-
Effects of gastrointestinal delivery of non-caloric tastants on energy intake: a systematic review and meta-analysis
European Journal of Nutrition (2021)
-
Reduced postprandial bone resorption and greater rise in GLP-1 in overweight and obese individuals after an α-glucosidase inhibitor: a double-blinded randomized crossover trial
Osteoporosis International (2021)
-
Investigation of the long-term sustainability of changes in appetite after weight loss
International Journal of Obesity (2018)