Abstract
OBJECTIVE: To assess the acute regulation of leptin concentrations by insulin, glucose and free fatty acids (FFAs).
DESIGN: Four protocols: saline control experiment (CON); hyperglycemic clamps (∼8.3 mmol/l, 120 min) after an overnight fast (12 FAST); after a 36 h fast (36 FAST); and after a 36 h fast during which Intralipid/heparin was given over the last 24 h (36 FAST+FFA).
SUBJECTS: Lean, young, healthy volunteers; control group (n=6), experimental group (n=6).
MEASUREMENTS: Serum leptin concentrations.
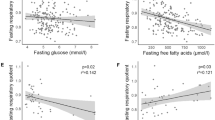
RESULTS: Glucose and insulin concentrations were similar during the three clamp protocols. Average FFAs during the last 60 min of the clamp were 671±68 µM (CON),109±15 µM (12 FAST), 484±97 µM (36 FAST) and 1762±213 µM (36 FAST+FFA). Leptin concentrations decreased similarly during 36 FAST and 36 FAST+FFA. Leptin concentrations at 120 min (expressed as percentage of mean basal value) were 0.82±0.02 (CON), 0.93±0.08 (12 FAST) (P=0.29), 1.19±0.06 (36 FAST) (P<0.01) and 1.44±0.12 (36 FAST+FFA) (P<0.01).
CONCLUSION: During a one-day fast leptin concentrations decrease regardless of maintainance of an isocaloric balance. During acute hyperinsulinemic hyperglycemia leptin concentrations increase only after a preceding fast. This increase was most pronounced during simultaneous elevation of FFAs. Overall, our findings are compatible with the hypothesis that leptin secretion may be coupled to triglyceride synthesis rather than to the absolute lipid content of the adipocyte.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue Nature 1994 372: 425–432.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F . Effects of the obese gene product on body weight regulation in ob/ob mice Science 1995 269: 540–543.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF . Serum immunoreactive-leptin concentrations in normal-weight and obese humans New Engl J Med 1996 334: 292–295.
Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL . Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels J Clin Endocrinol Metab 1997 82: 561–565.
Boden G, Chen X, Mozzoli M, Ryan I . Effect of fasting on serum leptin in normal human subjects J Clin Endocrinol Metab 1996 81: 3419–3423.
Grinspoon SK, Askari H, Landt ML, Nathan DM, Schoenfeld DA, Hayden DL, Laposata M, Hubbard J, Klibanski A . Effects of fasting and glucose infusion on basal and overnight leptin concentrations in normal-weight women Am J Clin Nutr 1997 66: 1352–1356.
Coleman RA, Herrmann TS . Nutritional regulation of leptin in humans Diabetologia 1999 42: 639–646.
Miell JP, Englaro P, Blum WF . Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects Horm Metab Res 1996 28: 704–707.
Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF . Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro Diabetes 1996 45: 699–701.
Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V . Physiological insulinemia acutely modulates plasma leptin Diabetes 1998 47: 544–549.
Utriainen T, Malmstrom R, Mäkimattila S, Yki-Järvinen H . Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects Diabetes 1996 45: 1364–1366.
Rentsch J, Chiesi M . Regulation of ob gene mRNA levels in cultured adipocytes FEBS Lett 1996 379: 55–59.
Deng C, Moinat M, Curtis L, Nadakal A, Preitner F, Boss O, Assimacopoulos Jeannet F, Seydoux J, Giacobino JP . Effects of beta-adrenoceptor subtype stimulation on obese gene messenger ribonucleic acid and on leptin secretion in mouse brown adipocytes differentiated in culture Endocrinology 1997 138: 548–552.
Peino R, Fernandez Alvarez J, Penalva A, Considine RV, Rodriguez-Segade S, Rodriguez-Garcia J, Cordido F, Casanueva FF, Dieguez C . Acute changes in free fatty acids (FFA) do not alter serum leptin levels J Endocrinol Invest 1998 21: 526–530.
Boden G, Chen X, Kolaczynski JW, Polansky M . Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects J Clin Invest 1997 100: 1107–1113.
Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Järvinen H, Evron W, Dailey G, Gerich J . Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM JAMA 1995 273: 1855–1861.
Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M . Radioimmunoassay of leptin in human plasma Clin Chem 1996 42: 942–946.
Stumvoll M, Fritsche A, Tschritter O, Lehmann R, Wahl HG, Renn W, Häring H . Leptin levels in humans are acutely suppressed by isoproterenol despite acipimox-induced inhibition of lipolysis, but not by free fatty acids Metabolism 2000 49: 335–339.
Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M . Plasma leptin and insulin relationships in obese and nonobese humans Diabetes 1996 45: 695–698.
Coppack SW, Persson M, Judd RL, Miles JM . Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue Am J Physiol 1999 276: E233–E240.
de la Llera M, Glick JM, Rothblat G . Mechanism of triglyceride accumulation in rat preadipocyte cultures exposed to very low density lipoprotein J Lipid Res 1981 22: 245–253.
Green H, Kehinde O . An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion Cell 1975 5: 19–27.
Saggerson ED, Greenbaum AL . The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue Biochem J 1970 119: 193–219.
Acknowledgements
We are indebted to Sabine Wolff for her excellent technical help. This study was in part supported by a grant from the Deutsche Forschungsgemeinschaft (DFG), Stu 192-2/1 and by a grant from the European Community (QLRT-1999-00674).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stefan, N., Fritsche, A., Häring, H. et al. Acute stimulation of leptin concentrations in humans during hyperglycemic hyperinsulinemia. Influence of free fatty acids and fasting. Int J Obes 25, 138–142 (2001). https://doi.org/10.1038/sj.ijo.0801527
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801527
Keywords
This article is cited by
-
Moringa oleifera leaf extract ameliorated high-fat diet-induced obesity, oxidative stress and disrupted metabolic hormones
Clinical Phytoscience (2019)
-
Comparative analysis of glucose metabolism responses of large yellow croaker Larimichthys crocea fed diet with fish oil and palm oil
Fish Physiology and Biochemistry (2019)
-
Modulation of hsa-miR-26b levels following adipokine stimulation
Molecular Biology Reports (2013)
-
2D-electrophoresis and multiplex immunoassay proteomic analysis of different body fluids and cellular components reveal known and novel markers for extended fasting
BMC Medical Genomics (2011)
-
Adipose Tissue as an Endocrine Organ
Obesity (2006)