Abstract
OBJECTIVE: The present study was performed to investigate the efficacy and safety of a caffeine/ephedrine (CE) mixture in obese adolescents.
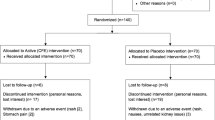
SUBJECTS: Thirty-two (m/f=16/16) obese children were included into the study. They were treated by diet (calculated daily energy requirement minus 500 kcal) and either CE or placebo (PL) for 20 weeks in a randomized double-blind placebo-controlled trial. Those weighing less than 80 kg took one tablet three times (100 mg/10 mg), whereas those weighing more than 80 kg took two tablets three times per day. There were three dropouts (girls) from the PL group. The age, weight body mass index (BMI) values (mean (range)) of the PL and CE groups were 16.0 (14.3–17.6) and 16.0 (14.2–17.7) y, 103.0 (77.2–126.4) and 104.8 (69.8–150.2) kg, 35.2 (28.3–42.3) and 36.5 (31.3–51.8) kg/m2, respectively.
RESULTS: The decrease in relative body weight, BMI and body fat (measured by bioelectric impedance) was significantly (P<0.05) greater in the CE group (mean±s.d.; 14.4±10.5%, 2.9±1.9 kg/m2, 6.6±6.0 kg) than in the PL group (2.2±5.8%, 0.5±1.6 kg/m2, 0.5±2.7 kg). Relative body weight decreased by more than 5% in 81% of the CE group, out only in 31% of the PL group. Adverse events were negligible and did not differ between the CE and PL groups. Withdrawal symptoms were mild, transient and their frequency and severity were not different between the placebo and active groups.
CONCLUSION: According to the present pilot study, CE can be a safe and effective compound for the treatment of obesity in adolescents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Csábi G, Török K, Jeges S, Molnár D . Presence of metabolic cardiovascular syndrome in obese children Eur J Pediatr 2000 159: 91–94.
Troiano RP, Flegal KM . Overweight children and adolescents: description, epidemiology, and demographics Pediatrics 1998 101 (Suppl): 497–504.
Astrup A, Toubro S, Hein P, Madsen J . Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study Metabolism 1991 40: 323–329.
Toubro S, Astrup AV, Breum L, Quaade F . Safety and efficacy of long-term treatment with ephedrine, caffeine and an ephedrine/caffeine mixture Int J Obes Relat Metab Disord 1993 17 (Suppl 1): S69–S72.
Daly PA, Krieger DR, Dulloo AG, Young JB, Landsberg L . Ephedrine, caffeine and aspirin: safety and efficacy fortreatment of human obesity Int J Obes Relat Metab Disord 1993 17 (Suppl 1): S73–S78.
Eiben O, Panto E . Body measurements in the Hungarian youth at the 1980's, based on the Hungarian National Growth Study Anthrop Közll 1987–1988 31: 49–68.
Wabitsch M, Braun U, Heinze E, Muche R, Mayor H, Teller W, Fusch C . Body composition in 5–18-y-old obese children and adolescents before and after weight reduction as assessed by deuterium dilution and bioelectrical impedance analysis Am J Clin Nutr 1996 64: 1–6.
Lusk G . The Elements of Science of Nutrition, 4th edn WB Saunders: Philadelphia, PA 1928.
Jones NL . Clinical Exercise Testing, 3rd edn WB Saunders: Philadelphia, PA 1988.
Astrup A, Breum L, Toubro S, Hein P, Quaade F . The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial Int J Obes Relat Metab Disord 1992 16: 269–277.
Pasqualy R, Cesary MP, Melchionda N, Stefanini C, Raitano A, Labo G . Does ephedrine promote weight loss in low-energy-adapted obese women? Int J Obes 1987 11: 163–168.
Astrup A, Buemann B, Christensen NJ, Toubro S, Thorbek G, Victor OJ, Quaade F . The effect of ephedrine/caffeine mixture on energy expenditure and body composition in obese women Metabolism 1992 41: 686–688.
Astrup A, Bülow J, Madsen J, Christensen NJ . Contribution of brown adipose tissue and skeletal muscle to thermo-genesis induced by ephedrine in man Am J Physiol 1985 248: E507–E515.
Stich V, Hainer V, Kunesova M . Effect of ephedrine/caffeine mixture on metabolic response to excercise in obese subjects Int J Obes Relat Metab Disord 1993 17 (Suppl 2): S31.
Acknowledgements
The project was supported by NYCOMED DAK Pharmaceutical Company, Roskilde, Denmark, and in part by the Hungarian National Research Fund (OTKA T-026663 to DM) and by the Hungarian Ministry of Welfare (081/1996 to DM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molnár, D., Török, K., Erhardt, E. et al. Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents. Int J Obes 24, 1573–1578 (2000). https://doi.org/10.1038/sj.ijo.0801433
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801433
Keywords
This article is cited by
-
Herbal medicine for sports: a review
Journal of the International Society of Sports Nutrition (2018)
-
Acute effects of a commercially-available pre-workout supplement on markers of training: a double-blind study
Journal of the International Society of Sports Nutrition (2014)
-
Pharmacotherapy for childhood obesity: present and future prospects
International Journal of Obesity (2013)
-
A randomized, triple masked, placebo-controlled clinical trial for controlling childhood obesity
World Journal of Pediatrics (2010)
-
ISSN exercise & sport nutrition review: research & recommendations
Journal of the International Society of Sports Nutrition (2010)