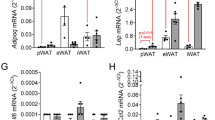
Abstract
OBJECTIVE: To investigate the effects of short term (15 days) cafeteria diet feeding on the expression of β3-AR in vivo and its association with lipolytic stimulation induced by β3-AR agonist CGP12177A in isolated white adipocytes.
ANIMALS: Six female and 6 male Wistar rats (at 4 weeks of age) were fed on a cafeteria diet plus standard diet for 15 days. The remaining 12 age- and sex-matched rats always received standard diet only.
MEASUREMENTS: White gonadal adipose tissue was isolated and used for the determination of β3-AR and leptin expression, and for in vitro studies of lipolytic activity.
RESULTS: Control male rats had higher levels of both β3-AR and leptin mRNA in white adipose tissue than their female counterparts. Both male and female rats up-regulated the levels of both β3-AR and leptin mRNA in response to 15 day cafeteria diet feeding. Noradrenaline- and isoprenaline-induced lipolysis were significantly increased in fat cells from control females compared to their male counterparts. CGP12177A stimulation resulted in significantly higher glycerol release in fat cells from cafeteria diet-fed female rats, whereas there were no differences due to dietary treatment in male rats. The maximal lipolytic response of forskolin (stimulating adenylyl cyclase) and dibutyryl cyclic AMP (cyclic AMP analogous) was not affected by sex or cafeteria diet feeding.
CONCLUSION: Cafeteria diet feeding brings about higher excess body weight and impaired adipose tissue lipolytic activity in female rats compared to male rats. Thus, the higher levels of β3-AR mRNA induced by cafeteria feeding are not indicative per se of an increase of the lipolytic response of the adipocytes. The changes seen in other adrenoceptor subtypes (β1 and β2) may be more determinant of the overall lipolytic response of adipocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lafontan M, Berlan M . Fat cell adrenergic receptors and the control of white and brown fat cell function J Lipid Res 1993 34: 1057–1091.
Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpéné C, Bousquet-Melou A, Berlan M . Adrenergic regulation of adipocyte metabolism Human Reprod 1997 12: 6–20.
Giacobino JP . β3-adrenoceptor: an update Eur J Endocrinol 1995 132: 377–385.
Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB . Targeted disruption of the β3-adrenergic receptor gene J Biol Chem 1995 270: 29483–29492.
Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM . An adipose tissue-specific β-adrenergic receptor. Molecular cloning and down-regulation in obesity J Biol Chem 1991 266: 24053–24058.
Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW . Impaired expression and functional activity of the β3- and β1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice Mol Endocrinol 1994 8: 518–527.
Howe R . β3-Adrenergic agonists Drugs Future 1993 18: 529–549.
Largis EE, Burns MG, Meunkel HA, Dolan JA, Claus TH . Antidiabetic and antiobesity effects of a highly selective β3-adrenoceptor agonist (CL316,243) Drug Dev Res 1994 32: 69–76.
Hashimoto K, Nagao Y, Ida K, Takeda M, Murakami N, Kato K, Mizota M . Improvement of metabolic disorders and visceral fat obesity by the β3-adrenoceptor agonist (R*, R*)-(±)-methyl-4-[2-[2-hydroxy-2-(3-chlorophenyl) ethylamino]propyl]-phenoxyacetate hydrobromide (BRL35135A) in genetically obese rodents Biochem Pharmacol 1996 52: 1529–1535.
Collins S, Daniel KW, Petro AE, Surwit RS . Strain-specific response to β3- adrenergic receptor agonist treatment of diet-induced obesity in mice Endocrinology 1997 138: 405–413.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue Nature 1994 372: 425–432.
Friedman JM, Halaas JL . Leptin and the regulation of body weight in mammals Nature 1998 395: 763–770.
Giacobino JP . Role of the beta3-adrenoceptor in the control of leptin expression Horm Metab Res 1996 28: 633–637.
Sclafani A, Springer D . Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes Physiol Behav 1976 17: 461–471.
Gianotti M, Roca P, Palou A . Body weight and tissue composition in rats made obese by a cafeteria diet. Effect of 24 hours starvation Horm Metab Res 1988 20: 208–212.
Lladó I, Picó C, Palou A, Pons A . Protein and amino acid intake in cafeteria fed obese rats Physiol Behav 1995 58: 513–519.
Roca P, Rodríguez AM, Oliver P, Bonet ML, Quevedo S, Picó C, Palou A . Brown adipose tissue response to cafeteria diet-feeding involves induction of the UCP2 gene and is impaired in female rats as compared to males Pflügers Arch 1999 438: 628–634.
Jacobsson A, Stadler U, Glotzer MA, Kozak LP . Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression J Biol Chem 1985 260: 16250–16254.
Rodbell M . Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis J Biol Chem 1964 239: 375–380.
Eggstein M, Kulhmann E . Triglycerides and glycerol. Determination after alkaline hydrolisis. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis, Vol 4: Academic Press: New York 1974, pp 1825–1831.
Motulsky HJ, Ransnas LA . Fitting curves to data using nonlinear regression: a practical and nonmathematical review FASEB J 1987 1: 365–374.
Giudicelli Y, Dieudonne MN, Lacasa D, Pasquier YN, Pecquery R . Modulation by sex hormones of the membranous transducing system regulating fatty acid mobilization in adipose tissue Prostaglandins Leukot Essent Fatty Acids 1993 48: 91–100.
Lafontan M, Berlan M . Fat cell α2-adrenoceptors: the regulation of fat cell function and lipolysis Endocr Rev 1995 16: 716–738.
Yagami T, Tohkin M, Matsubara T . The involvement of the stimulatory G protein in sexual dimorphism of beta-adrenergic receptor-mediated functions in rat liver Biochim Biophys Acta 1994 1222: 257–264.
Quevedo S, Roca P, Picó C, Palou A . Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue Pflügers Archiv 1998 436: 689–695.
Pecquery R, Dieudonne MN, Cloix JF, Leneveu MC, Dausse JP, Giudicelli Y . Enhancement of the expression of the alpha 2-adrenoreceptor protein and mRNA by a direct effect of androgens in white adipocytes Biochem Biophys Res Commun 1995 206: 112–118.
Rothwell NJ, Stock MJ . A role for brown adipose tissue in diet-induced thermogenesis Nature 1979 281: 31–35.
Masuzaki H, Ogawa Y, Hosoda K, Kawada T, Fushiki T, Nakao K . Augmented expression of the obese gene in the adipose tissue from rats fed high-fat diet Biochem Biophys Res Commun 1995 216: 355–358.
Granneman JG . Effects of agonist exposure on the coupling of beta 1 and beta 3 adrenergic receptors to adenylyl cyclase in isolated adipocytes J Pharmacol Exp Ther 1992 261: 638–642.
Carpéné C, Galitzky J, Collon P, Esclapez F, Dauzats M, Lafontan M . Desensitization of beta-1 and beta-2, but not beta-3, adrenoceptor-mediated lipolytic responses of adipocytes after long-term norepinephrine infusion J Pharmacol Exp Ther 1993 265: 237–247.
Acknowledgements
This work was supported by Dirección General de Enseñanza Superior e Investigación Científica of the Spanish Government (grant PM97-0094) and the European Commission (contract No. ERBCHRX© CT940490 and COST Action 918).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lladó, I., Estrany, M., Rodríguez, E. et al. Effects of cafeteria diet feeding on β3-adrenoceptor expression and lipolytic activity in white adipose tissue of male and female rats. Int J Obes 24, 1396–1404 (2000). https://doi.org/10.1038/sj.ijo.0801390
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801390
Keywords
This article is cited by
-
Isocaloric intake of a high-fat diet modifies adiposity and lipid handling in a sex dependent manner in rats
Lipids in Health and Disease (2011)
-
Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats
Nature Neuroscience (2010)
-
Gender Related Differences in Paraoxonase 1 Response to High‐fat Diet‐induced Oxidative Stress
Obesity (2008)
-
Sex‐differential Expression of Metabolism‐related Genes in Response to a High‐fat Diet
Obesity (2008)
-
PPAR‐γ2 Expression in Response to Cafeteria Diet: Gender‐ and Depot‐Specific Effects
Obesity Research (2004)