Abstract
OBJECTIVE: The objective of this study was to examine the influence of body weight and body composition on aspects of aerobic fitness. Our hypothesis was that increased body weight, specifically increased fat mass (FM), would not limit VO2max relative to fat-free mass (FFM), but would reduce maximal and sub-maximal VO2max relative to body weight.
DESIGN: We used data from two ongoing studies. In Study 1 a cross-sectional analysis of 129 children across a wide spectrum of body composition was performed. In Study 2 we examined data from 31 overweight women before and after weight loss.
METHODS: VO2max was measured using a treadmill test. Sub-maximal aerobic capacity was evaluated with respiratory exchange ratio (RER), heart-rate (HR), and oxygen uptake relative to VO2max at a given workload (%VO2max). Body composition was assessed using dual energy X-ray absorptiometry (DXA) (Study 1) and a four-compartment model (Study 2).
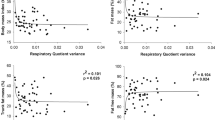
RESULTS: In Study 1, FFM was the strongest determinant of VO2max (r=0.87; P<0.0001). After adjusting for FFM, there was no significant influence of FM on VO2max. After separating children into lean and obese sub-groups, absolute VO2max was significantly higher in the obese (1.24±0.27 vs 1.56±0.40) and VO2max relative to body weight was significantly lower (44.2±3.2 vs 32.0±4.1 ml/(kg-min)), whereas there was no significant difference when expressed relative to FFM (57.9±5.8 vs 59.2±4.9 ml/(kgFFM-min)). Sub-maximal aerobic capacity was significantly lower in the obese children, as indicated by a higher HR and %VO2max; time to exhaustion was significantly lower in the obese children (15.3±2.9 vs 11.1±2.1 min). In Study 2, FFM was also the strongest determinant of VO2max before and after weight loss. The relationship between VO2max and FFM was identical before and after weight loss so that VO2max relative to FFM was identical before and after weight loss (43.8±4.9 vs 45.5±6.4 ml/(kgFFM-min)). However, sub-maximal aerobic capacity was lower in the obese state, as indicated by a significantly higher RER (0.85±0.06 vs 0.79±0.05), HR (124±14 vs 102±11 bpm), and %VO2max (44% vs 36%).
CONCLUSION: The major influence of body weight on VO2max is explained by FFM; FM does not have any effect on VO2max. Fatness and excess body weight do not necessarily imply a reduced ability to maximally consume oxygen, but excess fatness does have a detrimental effect on submaximal aerobic capacity. Thus, fatness and VO2max should be considered independent entities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blair SN, Kampert JB, Kohl III HW, Barlow LE, Macen LA, Paffenberger RS, Gibbons LW . Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women JAMA 1996 276: 205–210.
Farrell SW, Kampert JB, Kohl III HW, Barlow CE, Mocera CA, Paffenberger RS, Gibbons LW, Blair SN . Influences of cardiorespiratory fitness levels and other predictors on cardiovascular disease mortality in men Med Sci Sports Exerc 1998 30: 899–905.
Lee CD, Jackson AS, Blair SN . US weight guidelines: Is it also important to consider cardiorespiratory fitness? Int J Obes Relat Metab Disord 1998 22: S2–S7.
Lee CD, Blair SN, Jackson AS . Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men Am J Clin Nutr 1999 69: 373–380.
Blair SN, Kohl III HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW . Physical fitness and all-cause mortality a prospective study of healthy men and women JAMA 1989 262: 2395–2401.
Blair SN, Kohl III HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA . Changes in physical fitness and all-cause mortality JAMA 1995 273: 1093–1098.
McMurray RG, Ainsworth BE, Harrell JS, Griggs TR, Williams OD . Is physical activity or aerobic power more influential on reducing cardiovascular disease risk factors? Med Sci Sports Exerc 1998 30: 1521–1529.
Leger L . Aerobic performance in Docherty D (ed.) Measurement in pediatric exercise science Human Kinetics: Champaign 16: 1996, pp 183–223.
Boulay MR, Ama PF, Bouchard C . Racial variation in work capacities and powers Can J Sport Sci 1988 13: 127–135.
Rowland TW . Effects of obesity on aerobic fitness in adolescent females Am J Dis Child 1991 145: 764–768.
Zanconato S, Baraldi E, Santuz P, Rigon F, Vido L, DaDalt L, Zacchello F . Gas exchange during exercise in obese children Eur J Pediatr 1989 148: 614–617.
Reybrouck T, Weymans M, Vinckx J, Stijns J, Vanderschueren-Lodeweyckx M . Cardiorespiratory function during exercise in obese children Acta Paediat Scand 1987 76:: 342–348.
Mattsson E, Larsson UE, Rossner S . Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord 1997 21:: 380–386.
DeMeersman RE, Stone S, Schaefer DC, Miller WW . Maximal work capacity in prepubescent obese and nonobese females Clin Pediat 1985 24: 199–200.
Toth MJ, Goran Ml, Ades PA, Howard DB, Poehlman ET . Examination of data normalization procedures for expressing peak VO 2 data JAP 1993 75: 2282–2292.
Trowbridge CA, Gower BA, Nagy TR, Hunter GR, Treuth MS, Goran MI . Maximal aerobic capacity in African-American and Caucasian prepubertal children Am J Physiol 1997 273: E809–E814.
Franklin BA, Hellerstein HK, Gordon S, Timmis GC . Exercise testing: methods and protocols. In Wenger NK, Hellerstein HK (eds) Rehabilitation of the coronary patient Churchill Livingstone: New York 1992, pp 147–170.
Baumgartner RN, Heymsfield SB, Lichtman S . Body composition in elderly people: effect of criterion estimates on predictive equations Am J Clin Nutr 1991 53: 1345–1353.
Goran MI, Peters EJ, Herndon DN, Wolfe RR . Total energy expenditure in burned children using the doubly labeled water technique Am J Physiol 1990 259: E576–E585.
Wilmore JH . A simplified method for the determination of residual lung volume JAP 1969 27: 96–100.
Buskirk E, Taylor HL . Maximal oxygen intake and its relation to body composition, with special reference to chronic physical activity and obesity JAP 1957 11: 72–78.
Treuth MS, Figueroa-Colon R, Hunter GR, Weinsier RL, Butte NF, Goran MI . Energy expenditure and physical fitness in overweight vs non-overweight prepubertal girls Int J Obes Relat Metab Disord 1998 22: 440–447.
Maffeis C, Schutz Y, Schena F, Zaffanello M, Pinelli L . Energy expenditure during walking and running in obese and nonobese prepubertal children J Pediatr 1993 123: 193–199.
Farrell PA, Gustafson AB, Kalkhoff RK . Assessment of methods for assigning treadmill exercise workloads for lean and obese women Int J Obes 1985 9: 49–58.
Elliot DL, Goldberg L, Kuehl KS, Hanna C . Metabolic evaluation of obese and nonobese siblings J Pediatr 1989 114: 957–962.
Maffeis C, Zaffanello M, Zoccante L, Schutz Y, Pinelli L . Maximal aerobic power during running and cycling in obese and non-obese children Acta Paediatr 1994 83: 113–116.
Moody DL, Kollias J, Buskirk ER . Evaluation of aerobic capacity in lean and obese women with four test procedures J Sports Med 1969 9: 1–9.
Cooper DM, Poage J, Barstow TJ, Springer C . Are obese children truly unfit? Minimizing the confounding effect of body size on the exercise response J Pediatr 1990 116: 223–230.
Davies CTM, Godfrey S, Light M, Largeant AJ, Zeidifard E . Cardiopulmonary responses to exercise in obese girls and young women JAP 1975 38: 373–376.
Huttunen NP, Paavilainen T . Physical activity and fitness in obese children Int J Obes 1986 10: 519–525.
Epstein LH, Koeske R, Zidansek J, Wing RR . Effects of weight loss on fitness in obese children Am J Dis Child 1983 137: 654–657.
Welsman JR, Armstrong N . The measurement and interpretation of aerobic fitness in children: current issues J R Soc Med 1996 89: 281P–285P.
Katch VL . Use of the oxygen/body weight ratio in correlational anaylses: spurious correlations and statistical considerations Med Sci Sports Exerc 1973 5: 253–257.
Winter EM . Partitioning out differences in size Pediatr Exerc Sci 1992 4: 296–301.
Vanderburgh PM, Katch FI . Ratio scaling of VO2max penalizes women with larger percent body fat, not lean body mass Med Sci Sports Exerc 1996 28: 1204–1208.
Nevill AM, Ramsbottom R, Williams C . Scaling physiological measurements for individuals of different body size Eur J Appl Physiol 1992 65: 110–117.
Welsman JR, Armstrong N, Nevill AM, Winter EM, Kirby BJ . Scaling peak VO2 for differences in body size Med Sci Sports Exerc 1996 28: 259–265.
Janz KF, Burns TL, Witt JD, Mahoney LT . Longitudinal analysis of scaling VO2 for differences in body size during puberty: the Muscatine Study Med Sci Sports Exerc 1998 30: 1436–1444.
Heil DP . Body mass scaling of peak oxygen uptake in 20- to 79-yr-old adults Med Sci Sports Exerc 1997 29: 1602–1608.
Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB . Statistical considerations regarding the use of ratios to adjust data Int J Obes Relat Metab Disord 1995 19: 644–652.
Tanner JM . Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation JAP 1949 2: 1–15.
Astrand PO, Rodhl K . Body dimensions and muscular exercise. In Astrand PO, Rodahl K (eds) Textbook of work physiology McGraw-Hill: New York 1986, pp 391–411.
Acknowledgements
We thank Tena Hilario, Paul Zuckerman, William Vaughn, and Betty Darnell for their enthusiastic work on this project. We are also grateful to the women and children who committed their time to this study. For the weight loss study, Stouffer's Lean Cuisine entrées were kindly provided, free of charge, by the Nestlé Food Company, Solon, OH.
This work was supported by the United States Department of Agriculture (95-37200-1643), The National Institute of Child Health and Human Development (RO1 HD/HL 33064), (RO1 DK 49779-03), and in part by a General Clinical Research Center grant (MO1-RR-00032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goran, M., Fields, D., Hunter, G. et al. Total body fat does not influence maximal aerobic capacity. Int J Obes 24, 841–848 (2000). https://doi.org/10.1038/sj.ijo.0801241
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801241
Keywords
This article is cited by
-
Impaired cardiorespiratory and neuromuscular fitness in children and adolescents with juvenile idiopathic arthritis: a cross-sectional case–control study in the era of biologic drug therapies
Pediatric Rheumatology (2023)
-
Cardiac output and arteriovenous oxygen difference contribute to lower peak oxygen uptake in patients with fibromyalgia
BMC Musculoskeletal Disorders (2023)
-
Associations between cardiorespiratory fitness and cardiometabolic risk factors in children and adolescents with obesity
Scientific Reports (2023)
-
Epicardial fat and Stage B heart failure among overweight/obese and normal weight individuals with diabetes mellitus
The International Journal of Cardiovascular Imaging (2023)
-
Is BMI Associated with Cardiorespiratory Fitness? A Cross-Sectional Analysis Among 8470 Apparently Healthy Subjects Aged 18–94 Years from the Low-Lands Fitness Registry
Journal of Science in Sport and Exercise (2022)